Consensus community valuation
Straws are discrete research notes that relate to a particular aspect of the company. Grouped under #hashtags, they are ranked by votes.
A good Straw offers a clear and concise perspective on the company and its prospects.
Please visit the forums tab for general discussion.
As I’m a bit over getting bullied by spec resources investors (*cough* @BkrDzn), I thought it might be worthwhile to filter out the gold price for a while and focus on an interesting microcap idea.
Like most microcaps that that have been listed for a while, Nova Eye Medical (EYE) comes with a rocky history. I won’t go too deeply into it given our friendly LLM junior analysts can do a pretty good job of summarising. But long story short EYE is the old Ellex Medical Lasers, who after selling their laser business to a French company in 2020, used the proceeds to fund the development and commercialisation of iTrack, a microcatheter used in canaloplasties of the eye to treat glaucoma. Messy history aside, EYE now appears to be a one product company and a much cleaner investment story.
The treatment of glaucoma is a big industry but is dominated by eye drops which make up 85-90% of the market. That said, minimally invasive glaucoma surgeries (MIGS) are growing, largely because a lot of patients are non-compliant with eye drops and glaucoma is a progressive disease where they eventually require surgical intervention. Improvements in MIGS technologies is also leading to many ophthalmologists to prefer surgical intervention sooner.
Within the smaller but growing MIGS segment the dominant solution is bypass stents to reduce the build-up of pressure in the eye. Stents have historically been 60-70% of the MIGS market, but with improvements in products and a preference for ophthalmologists to not leave hardware behind in the eye, canaloplasties are taking market share.
EYE’s original iTrack device had FDA approvals since 2008, but it wasn’t until the approval and commercialisation of the second generation iTrack Advance in 2023 where we started to see good sales traction, particularly in the US:
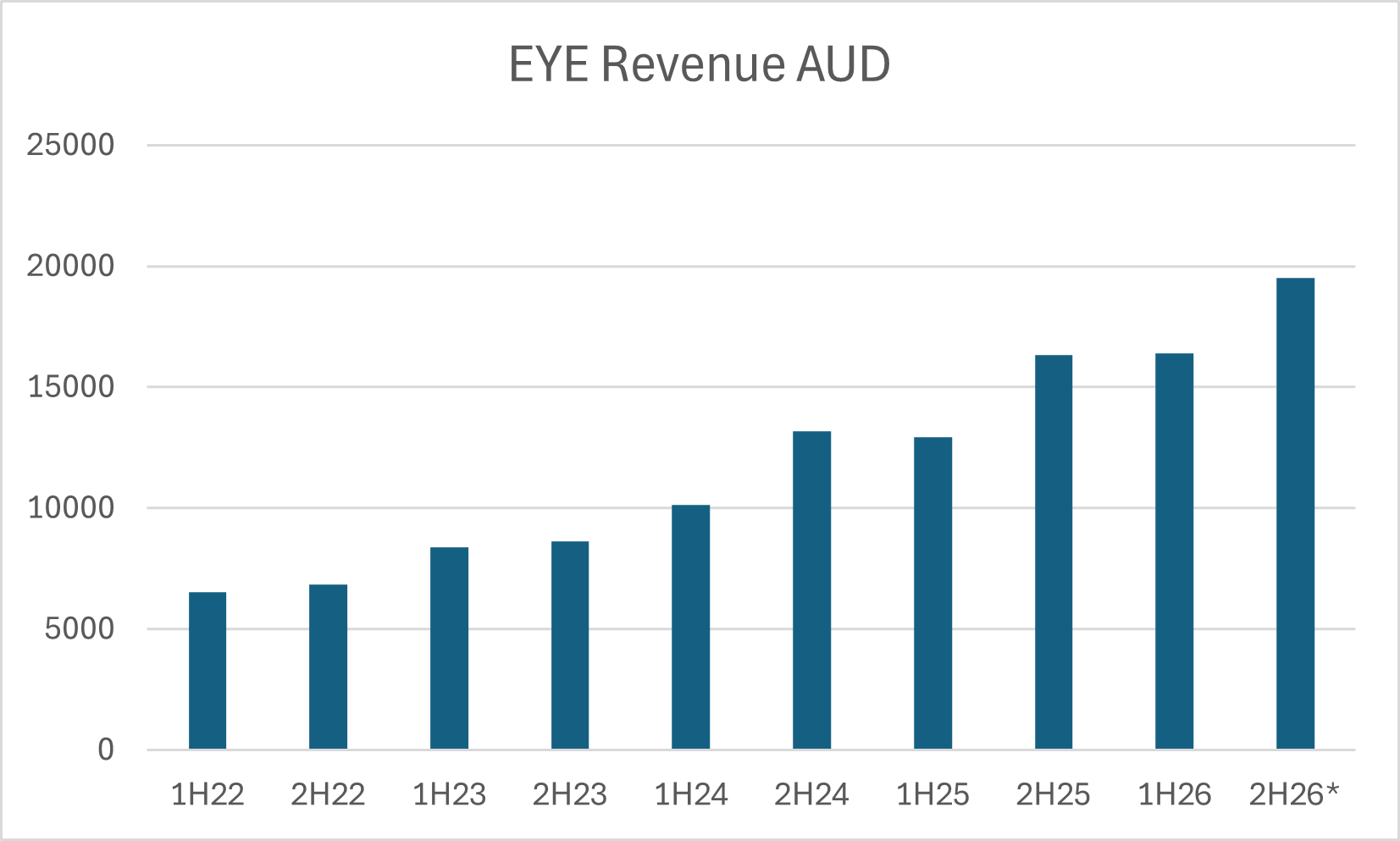
In the canaloplasty niche EYE effectively has just one competitor which is the Omni system produced by US based Sight Sciences. Fortunately, Sight Sciences is a listed business and we can track their glaucoma treatment segment over time and see how it compares to EYE:
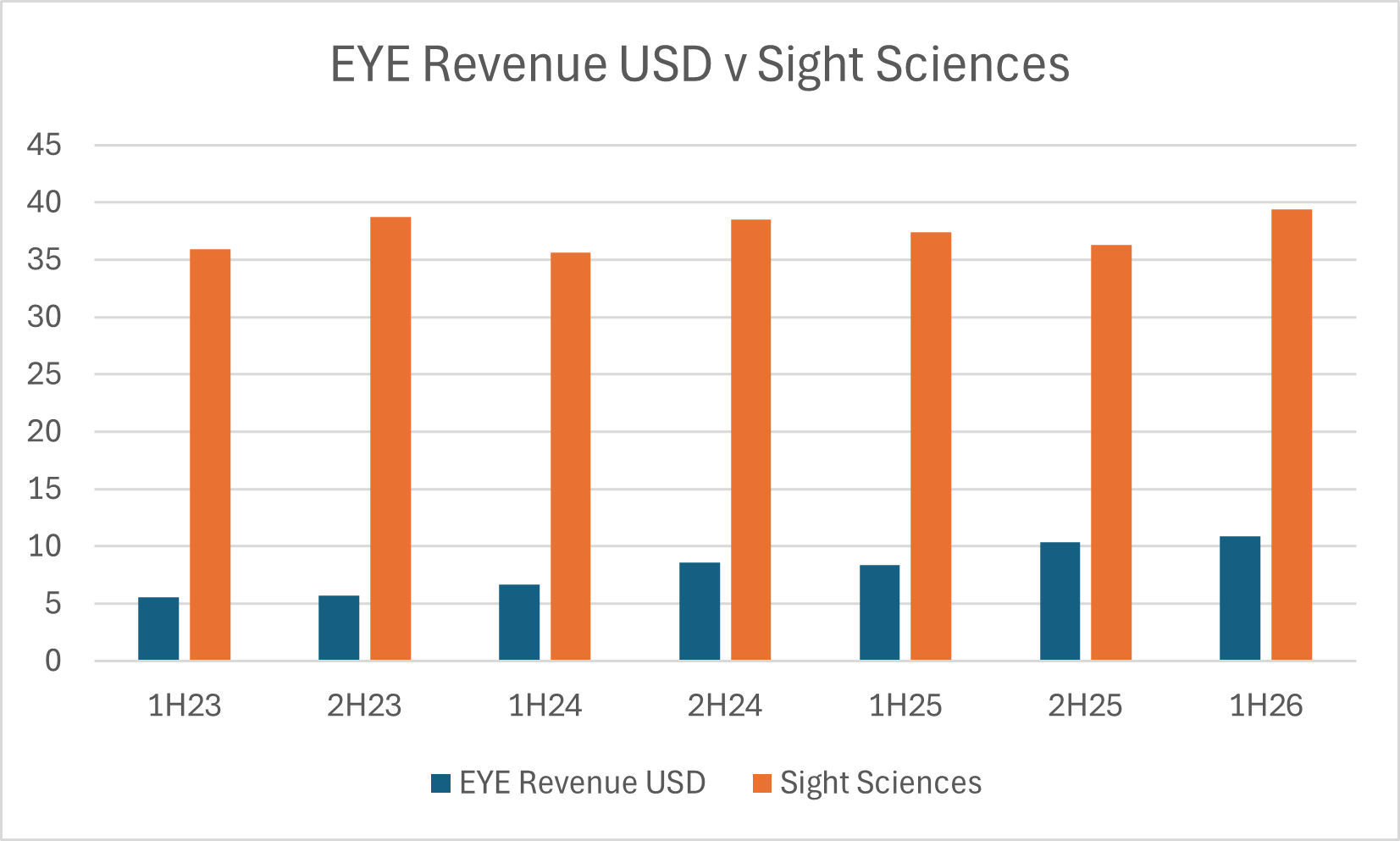
Yes, it’s off a low base, but nonetheless it’s impressive to see EYE ~2x its revenue while Sight Sciences has largely been flat at the same time. It also supports EYE’s claim to be growing 3x faster than the broader glaucoma market.
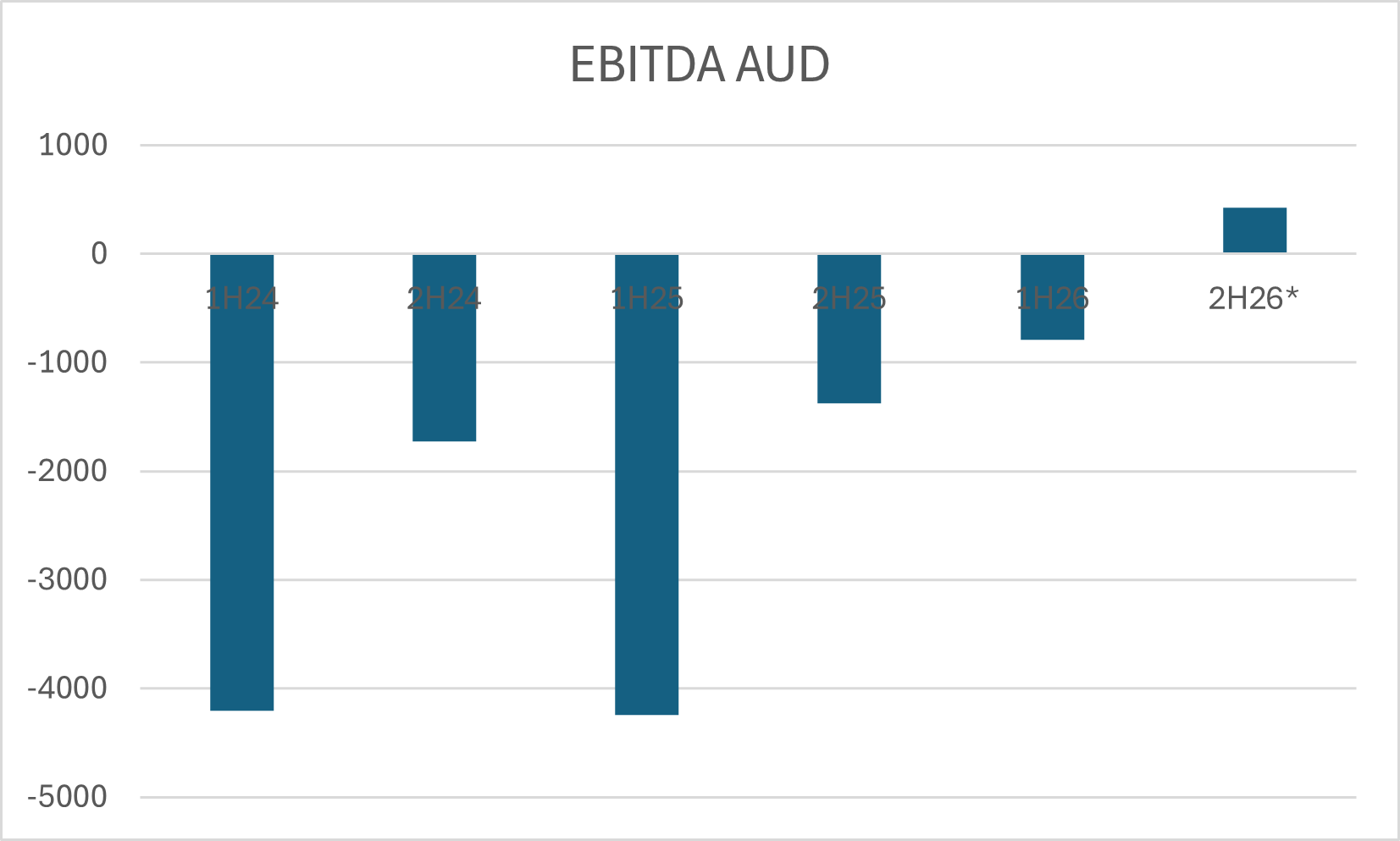
But we know what the market has wanted from microcaps for quite some time and that is profitability (or a clear path towards it). On that front EYE management has guided to EBITDA breakeven in 2H26, though it is worth noting that in the past this is a business/management that has missed targets and ultimately disappointed investors. That said, they have done a decent job in recent years of maintaining cost control while revenue has grown. It’s noteworthy that administration/corporate costs have been steady/falling with modest increases coming from sales/R&D:
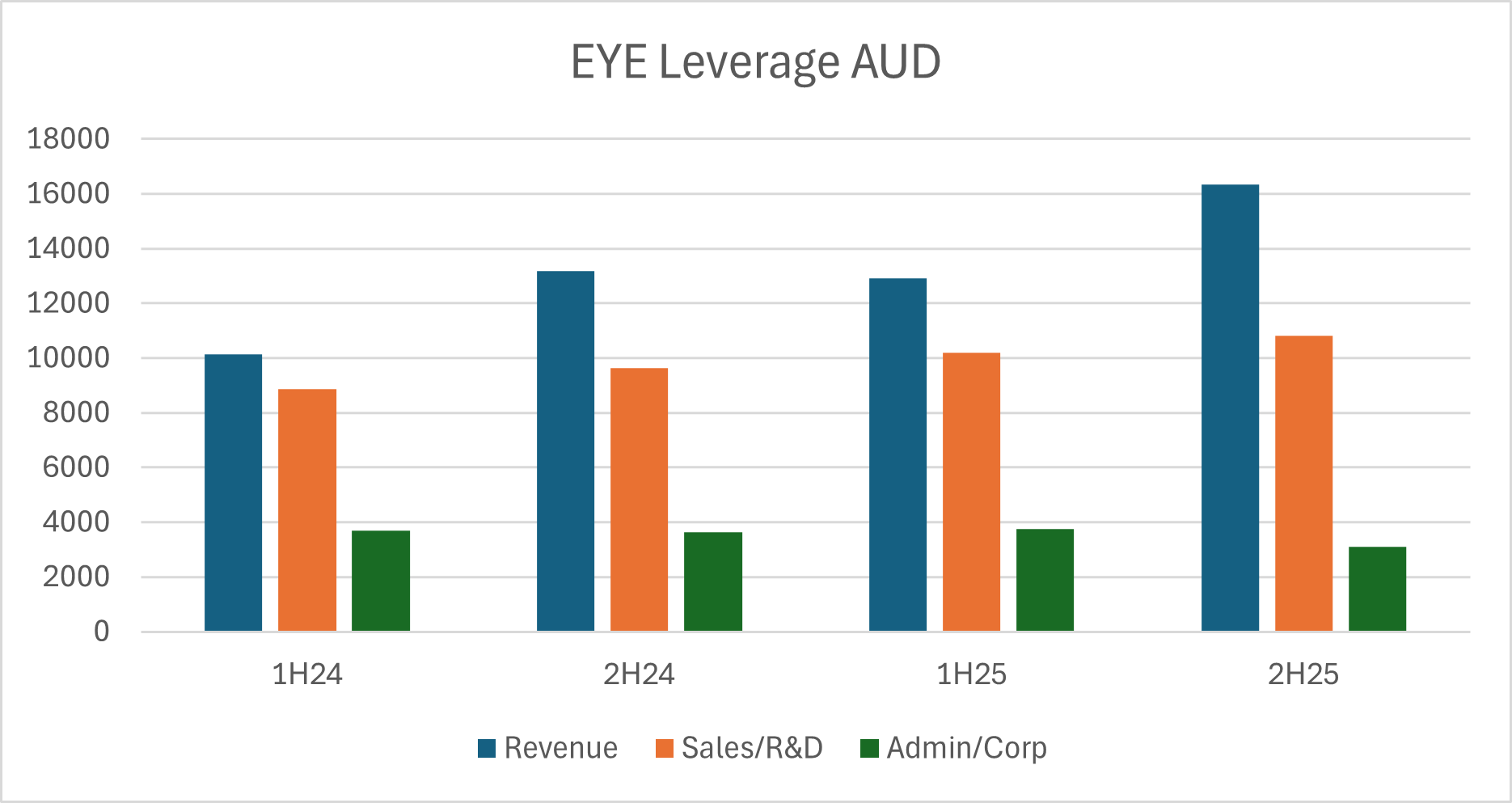
Assuming these cost trends continue and management land in the upper end of their guidance range, I think the target of EBITDA breakeven is achievable and would be a huge turnaround from the heavy operating losses of a couple of years ago:
Of course, despite being an interesting idea EYE is not short of risks. While they have shown consistently strong execution in their US sales since the launch of iTrack Advance, they need to continually invest in sales and R&D to maintain an advantage over larger peers with more resources. Changes to reimbursement is also a risk, right now reimbursement is $542 for surgeons and $2,231 for facilities which is supportive for MIGS growth, but reimbursement rates can change.
Finally, liquidity risk is real. EYE is tracking toward EBITDA profitability, but there is ~$1m in cash costs below that line in capitalised R&D and leases. At September EYE had $4m cash in the bank and a $2m working capital facility. The line between current cash liquidity and sustainable free cashflow breakeven is a tightrope, not impossible but it will require continued sales execution towards the top end of current guidance.
Ultimately, I think EYE is at an interesting point from an investment lens. A product gaining traction in the lucrative US market with high gross margins that can lead to genuine operating profit leverage as revenue scales over modest sales/R&D cost growth. The risk as we approach this key inflection is if US sales can't maintain its >20% revenue growth rate given the balance sheet may not be able to support any hiccups.
Would also love to get a view from the doyens of bio/medtech @mikebrisy and @Scoonieon this one!
Nova Eye pioneered the fastest growing segment in the burgeoning ophthalmic market; canal surgery for glaucoma, and holds over 100 patents in this domain
Nova Eye has invested in developing a next generation product (iTrackTM Advance)
The iTrack advance gained FDA approval of Friday 31 Mar. Shares jumped around 25% initially then pulled back for a 5% gain.
I notice there was some activity on strawman a few years ago. Perhaps some strawfolks are able to comment on these new developments;) With the original device still in use the new design should gain widespread adoption fairly soon and it will be interesting to hear progress on the rollout and uptake


Held SM and IRL
Nova Eye Medical Limited (ASX: EYE) #bear case #excellent licence plate
#best ticker code on the ASX
Thanks to Strawman and members @GnomeofZurich and @Bear77 I had a shallow dive in to Nova Eye. Although I love the ticker code I would be wary of the stock.
EYE looks like a day traders play thing. A quick 5 year ASX glance shows a stock with highs and promise of potential of $1.58 per share in 2016. A slow decline saw a drop to lows of 0.30c in July 2021.
But wait…..
An exciting, heart pumping upswing currently to 0.42c per share.
Let’s dive deeper……
Founded 1985 Nova Eye has sales headquarters in Freemont California and Dunedin NZ. The company maintains 12% Board ownership (a plus but not huge).
What does Nova Eye Medical Ltd Do?
Manufacture, service and distribute medical equipment and devices to diagnose and treat eye disease.
Company Numbers
Market cap $61.3 M
Shares on issue 144 Million
Trading volumes 120,000 – 250,000 share / day
Cash on Hand $17.8 M
EPS $0.256
NTA $0.18
Claims of TAM for products of US $5.9 Billion for glaucoma devices and approximately US $5.1 Billion for competition to age related macular degeneration (AMD) injections.
Revenue (shows slow growth)
$13.6 M 2021 up from
$12.8 M 2020
Only 8% increase
EBIT loss over the last year
Burnt through $15.5 M and made loss of $-4.4 M
Drum roll……I smell a capital raise in the next 12 months.
Debt but no Earnings
$3.7 M debt 2021 up from
$1.55 M debt in 2020
Cash on Hand
$17.8 M on hand
Net cash position of $14.1 M (less liabilities of $4.69M and upcoming liability of $1.97M)
Long and short of it : losing money
Recent Uptick
As far as I can tell recent share price spike as @GnomeofZurich rightly pointed-out is linked to announcement of an acquisition of a US portfolio of glaucoma device patents (August 2021).
EYE paid $2M plus $1.7M fully paid shares (under escrow for 12 month)
Business Offerings
2 Streams of product revenue / future anticipated revenue
1) Glaucoma medical devices
2) AMD/ 2RT nanosecond laser
Stream 1: Glaucoma treatment devices
Glaucoma is the second leading cause of blindness worldwide affects 300, 000 Australians
And estimates suggest it affects between 60-130 million people world-wide.
Stream 2: Age Related Macular Degeneration (AMD) specifically intermediate type treatment device.
(AMD) is the leading cause of blindness worldwide affecting 1.4 million Australians. Estimated 14% of Americans over 80 years.
Let’s expand……
Glaucoma what NOVA EYE offers
Total Addressable Market (TAM) of US $5.9B for these devices
Recent patent purchase from US saw Molteno3 glaucoma drainage device coming into Nova Eyes product fold.
Molteno3 is one of the most frequently used glaucoma drainage devices in the world. One of the “gold standards” for the treatment of advanced primary open angle glaucoma. A last resort treatment to save remaining sight.
Glaucoma is an irreversible blinding disease caused by optic nerve damage. This is slow and often asymptomatic until well advanced. Patients lose their peripheral vision slowly.
Lay person terms:
• You can think of the eye as a small pump
• We produce fluid to keep the eye inflated so it can work, just like we need air inside a car tyre
• There is a primary drainage angle that sits like a 360 degree gutter in front of the colored part of the eye (the iris), in patients with glaucoma the eye produces more fluid than it can drain through this gutter leading to an outflow imbalance and eventual high intraocular backflow pressure that damages the optic nerve (the plug that connects the eyeball to the brain).
• Sometimes debris from inside the eye can build up in the drainage angle – think of the angle like a gutter on a roof, occasionally leaves can get caught in the gutter so fluid can’t drain away as easily
So what does the Molteno3 device do?
This surgically implanted device sits underneath the white of the eye (the conjunctiva) and part of it (a tube) sits inside the eye allowing the fluid inside the eye to bypass any compromised natural drainage tissue and exit the eye more easily. This lowers eye backflow pressure and stops damage to the delicate cables that run around the back of the eye.
This device has excellent long-term results in studies with up to 88% success rates for the past 20 years of use. It really does help to lower eye pressure and preserve vision.
However it is a complex surgery and requires intensive follow because of risk of serious complications.
So is this acquisition a BIG DEAL? …. Well YES and NO!
Molteno3 while a “gold standard” device has a great deal of competition. Think of the TAM being sliced like a pie and Molteno3 really is only a small and decreasing sliver.
Competition is steep in glaucoma device land
This is the kicker for me and my bear case for this revenue stream of Nova Eye.
Molteno3 has a direct and equally effective competitor in the advanced glaucoma drainage space called the Baerveldt tube.
Baerveldt glaucoma device is sold by Johnson and Johnson. Studies show that although surgeons take slightly longer to implant it (roughly 13 minutes longer) it is equally effective in patient outcomes. AKA lowering eye pressure and preserving sight.
Other slivers of the glaucoma device pie
SLT (selective laser trabeculoplasty)
Glaucoma device technology has improved significantly over the past decade. Glaucoma patients are frequently screened better in practice so disease is detected earlier.
Initial treatment alternatives are also really effective at preventing progression to advanced glaucoma and the need from drainage implants like Molteno3.
The LIGHT study published in the LANCET in 2019 is a glaucoma treatment game changer. Patients with high pressure and glaucoma were found to benefit from an initial treatment of laser to this drainage angle as a first line treatment. This is instead of medicated drops. This can prevent disease progression.
One hit of laser can last up to 5 years with a repeat possible, giving a possible 10 year eye pressure lowering cover.
MIGS devices (minimally invasive glaucoma surgery)
MIGS are surgical implants but much easier for surgeons to place. They also work by helping the fluid or aqueous to flow out of the eye. Glaukos is one of the main companies producing this tech. Major devices include the Istent and Hydrus. These devices are inserted into the eye drainage tissue to help outflow.
Increasingly popular these devices provide a 30% reduction in IOP. The benefits of these devices last at least 5 years and likely longer. Patients don’t progress as readily and don’t need major glaucoma drainage surgery. So the Molteno3 is hopefully going to be needed less in the future.
NOVA EYE and the ITrack (Other glaucoma device offerings)
Itrack is a patented MIGS offering by Nova Eye. It is a micro catheter implant that feeds 360 degree into the tube like drainage outflow channel (Schlemm’s canal). That gutter system we were talking about. This is new technology in the MIGS space.
My concern is that there is limited data on efficacy. It also purports to lower eye pressure by 30%. Nova Eye claims that it doesn’t damage internal eye tissue like other MIGS devices do. This means that if glaucoma does get worse and further surgeries are needed there is more viable tissue to tap into to improve outflow pathways.
Studies are small and I am dubious of the claims so far and suggest the surgery looks more difficult than the Istent or Hydrus due to the accuracy needed to feed the tube through.
This tech doesn’t significantly increase aqueous fluid outflow compared to competitive market devices.
There are early claims that the inside lining of the cornea (the endothelium) is preserved better but very limited evidence for this claim. I am failing to see a competitive advantage of this MIG device - yet. But happy to be proven wrong.
The known unknowns
Pharmaceutical and device companies around the world are racing to develop the next latest greatest device. Protection of the eye cables in the retina with new therapies is a likely gamechanger in the future which may render outflow devices prehistoric.
Stream 2: AMD intermediate treatment device
Macular degeneration is an eye disorder that affects people over 50. There are 2 major types wet and dry macular degeneration. Both involve the progressive loss of central vision due to damage of the underlying retinal tissue.
Wet AMD is treatable. Wet implies leaky new blood vessels that develop in the retinal tissues. Eye injections are used to improve and maintain central vision. Injections include Eyelea and Avastin. Once diagnosed with wet AMD a patient is on a regime of injections spaced every 6 to 12 weeks for the rest of their lives. Or until they no longer experience a sight benefit.
Eye injection drug technology is a very hot game with the likes of Australian companies such as OPTHEA (OPT) in this space. The goal is to find a treatment that works effectively and allows for fewer and fewer injections.
Wet AMD is a veritable gold mine. Most Ophthalmologists charge around $312 ++ per injection and the Medicare rebate is around $266 from the government. This is per injection. Every time.
Currently there is no effective treatment for dry AMD.
Let’s find out about the 2RT laser
The second major product offering from Nova Eye is a world first nanosecond laser therapy 2RT for macular degeneration. This product plays in the AMD space.
The laser treatment is only effective on intermediate type macular degenerative changes. The laser is pulsed for a tiny duration to prevent damage and heating of surrounding tissue. The pulse is purported to have regenerative cell properties.
2RT is cleared for use in Europe and Australia but is still waiting on FDA approval.
The laser is meant to stop dry intermediate AMD from converting to wet AMD or at least delaying the change. This reduces the number of injections needed.
Nova Eye reports success of the 2RT laser tech based on the LEAD study (a randomized controlled trial). People have raised concern about this study. The extent of success and reduction of progression of wet AMD was based on post hoc analysis removing a certain population type of people from the study results.
I am always wary of post hoc statistical reporting. Oh! Those weren’t the results I wanted…… wait……if I remove these people or that group suddenly things are way more rosey.
The reality is that a whole group of patient’s didn’t show the same benefit from the laser if they had a certain type of macular tissue deposit at a certain depth.
I am currently unaware of any colleagues using the 2RT technology for treatment of AMD.
I would personally wait for FDA approval of the 2RT, further uptake of this method and repeated studies delineating patients into class of AMD before getting too excited.
Summary
· Cool Ticker coder
· Loss making company
· Slow growth
· Debt increasing
· High cash burn
· Need for upcoming capital injection
· I feel recent purchase US glaucoma patent portfolio isn’t spectacular
· 2RT tech doesn’t have enough evidence based support for me to get too excited
· I feel the company is overhyped based on recent acquisition
23-Dec-2020: Ord Minnett: Nova Eye Medical (EYE): Renewed iTrack sales growth points to a strong future
Analyst: Dr DENNIS HULME, [email protected] +612 9377 1500 www.taylorcollison.com.au
- Speculative Investment
- Recommendation: Outperform
- Market Capitalisation: $49M
- Pro forma cash after capital return: $26.5m
- Share price: $0.34
- 52 week low: $0.285
- 52 week high: $0.465
- OM Valuation: $0.42/share
Nova Eye Medical (Nova) is focused on its high-growth glaucoma franchise following the sale of the Ellex laser and ultrasound business in June. The disposable iTrack catheter used in minimally invasive glaucoma surgery (MIGS) has been a key driver of revenue growth, delivering a sales CAGR of 31.4% from FY15 to FY19. While iTrack sales faltered in FY20, it has returned to its growth path in recent months, with sales in the 4 months ending 31 October 2020 13% higher than the previous corresponding period. Nova has expanded its glaucoma product range with the acquisition in July of the highly regarded but under-promoted Molteno3 glaucoma drainage implant, which will benefit from Nova’s sales and marketing infrastructure. Nova will seek a partner to support further development of its 2RT laser for treating intermediate age-related macular degeneration (iAMD). It has a strong balance sheet, retaining $26.5m in cash from the sale of the laser and ultrasound business, so it is well positioned to capitalise on organic and inorganic growth opportunities. We initiate coverage with an Outperform recommendation and a valuation of $0.42/share.
--- click on the link above for the full report ---