Consensus community valuation
Straws are discrete research notes that relate to a particular aspect of the company. Grouped under #hashtags, they are ranked by votes.
A good Straw offers a clear and concise perspective on the company and its prospects.
Please visit the forums tab for general discussion.
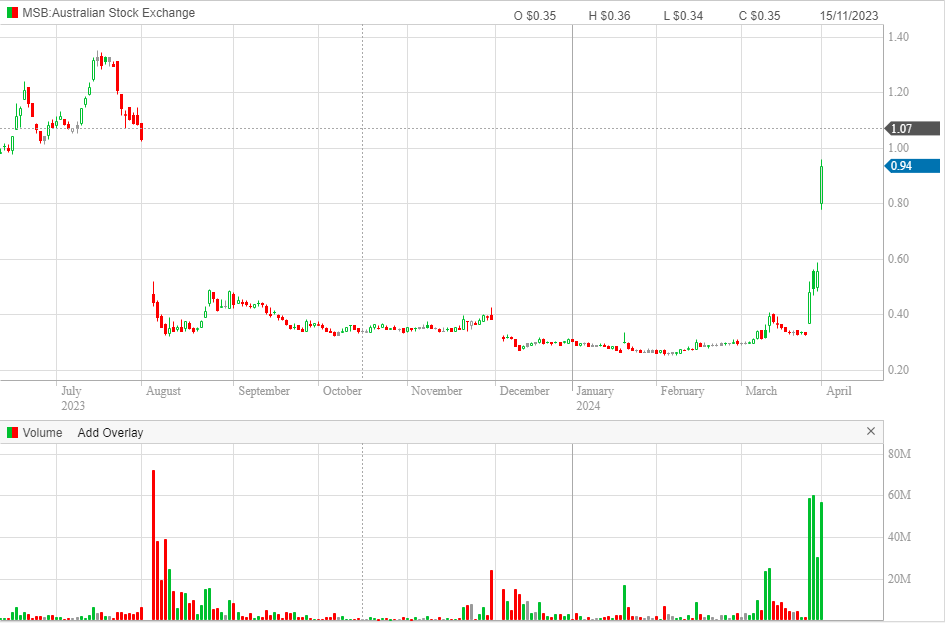
Shares in Mesoblast are up ANOTHER 70%-odd today. Shares have seen a 3x gain since it announced last week that the FDA would allow the company to re-submit the Biologics Licence Application for its Ryoncil product due to new clinical data (the company had unsuccessfully submitted for the licence twice before).
I have no insight into the company, the product or the opportunity -- but what a move!
POSITIVE OUTCOMES OF FIRST CHILDREN TREATED WITH REMESTEMCEL-L FOR MULTISYSTEM INFLAMMATORY SYNDROME (MIS-C) AND HEART FAILURE POSTCOVID-19 PUBLISHED IN PEDIATRICS
Melbourne, Australia; February 17, and New York, USA; February 16, 2021: Mesoblast Limited (ASX:MSB; Nasdaq:MESO), global leader in allogeneic cellular medicines for inflammatory diseases, today announced that Pediatrics (Journal of the American Academy of Pediatrics) has published a paper on the first two children treated with Mesoblast’s mesenchymal stromal cell (MSC) product candidate remestemcel-L for life-threatening multisystem inflammatory syndrome (MIS-C) associated with COVID-19.
The manuscript, titled ‘Remestemcel-L Therapy for COVID-19-Associated Multisystem Inflammatory Syndrome in Children,’ was based on two children admitted to the Medical University of South Carolina’s MUSC Shawn Jenkins Children’s Hospital, who were the first ever to be treated with remestemcel-L for MIS-C. Its authors include Allison Ross Eckard, MD, Professor of Pediatrics and Medicine and Division Chief of Infectious Diseases and Dr. Andrew M. Atz, Professor and Chair of the Department of Pediatrics at the Medical University of South Carolina. The article can be accessed at https://doi.org/10.1542/peds.2020-046573
MIS-C, a potentially life-threatening inflammatory condition which involves multiple critical organs and their vasculature, is associated with prior rather than active COVID-19 infection. It is thought to be a post-viral autoimmune process where the body’s over-zealous reaction to the virus causes the damage, rather than the virus itself. In approximately 50% of cases this inflammation is associated with significant cardiovascular complications resulting in decreased heart function and the presence of clinically important cardiovascular symptoms.1-3
MESOBLAST PHASE 3 TRIAL SHOWS THAT A SINGLE INJECTION OF REXLEMESTROCEL-L + HYALURONIC ACID CARRIER RESULTS IN AT LEAST TWO YEARS OF PAIN REDUCTION WITH OPIOID SPARING ACTIVITY IN PATIENTS WITH CHRONIC LOW BACK PAIN DUE TO DEGENERATIVE DISC DISEASE
Mesoblast Limited (ASX:MSB; Nasdaq:MESO), global leader in allogeneic cellular medicines for inflammatory diseases, today announced results from the Phase 3 randomized controlled trial of its allogeneic mesenchymal precursor cell (MPC) therapy rexlemestrocel-L in 404 enrolled patients with chronic low back pain (CLBP) due to degenerative disc disease (DDD) refractory to conventional treatments. The results indicate that a single injection of rexlemestrocel-L may provide a safe, durable, and effective opioidsparing therapy for patients with chronic inflammatory back pain due to degenerative disc disease, and that greatest benefits are seen when administered earlier in the disease process before irreversible fibrosis of the intervertebral disc has occurred.
“The durable pain reduction for at least two years from a single administration indicates that rexlemestrocel-L has the potential to change the treatment paradigm for chronic low back pain due to inflammatory disc disease, a condition that affects as many as seven million patients across the United States and Europe, and to prevent or reduce opioid use and dependence” said Dr Silviu Itescu, Chief Executive Officer of Mesoblast.
DISC: Previously held
I would ask existing and prospective shareholders to go back and read the annual reports for the past 15 years.
Management uses the same playbook over and over:
- Find blue sky opportunity
- Promise it's close
- Fail
- Repeat
Everytime they raise money, they invite institutional shareholders to Rockpool (a nice Neil Perry restaurant) in Sydney to eat a lavish lunch, using money raised from shareholders.
Mesoblast's superpower is to find different pools of capital to tap, for the past decade. It has yet to learn how to make money for shareholders.
Management drink cocktails made from the tears of past shareholders.
It is impressive they continue to find new pools of money to burn.
02-Oct-2020: Update on BLA for Graft Versus Host Disease
MESOBLAST RECEIVES COMPLETE RESPONSE LETTER FROM THE FDA FOR BIOLOGICS LICENSE APPLICATION FOR STEROID-REFRACTORY ACUTE GRAFT VERSUS HOST DISEASE IN CHILDREN
Mesoblast Limited (ASX:MSB; Nasdaq:MESO), global leader in allogeneic cellular medicines for inflammatory diseases, announced today that the US Food and Drug Administration (FDA) has issued a Complete Response Letter to its Biologics License Application (BLA) for remestemcel-L for the treatment of pediatric steroid-refractory acute graft versus host disease (SR-aGVHD). While the Oncologic Drugs Advisory Committee (ODAC) of the FDA voted 9:1 that the available data support the efficacy of remestemcel-L in pediatric patients with SR-aGVHD, the FDA recommended that Mesoblast conduct at least one additional randomized, controlled study in adults and/or children to provide further evidence of the effectiveness of remestemcel-L for SR-aGVHD.
As there are currently no approved treatments for this life-threatening condition in children under 12, Mesoblast will urgently request a Type A meeting with the FDA, expected within 30 days, to discuss a potential accelerated approval with a post-approval condition for an additional study.
--- click on link above for the remainder of this announcement ---