Consensus community valuation
Straws are discrete research notes that relate to a particular aspect of the company. Grouped under #hashtags, they are ranked by votes.
A good Straw offers a clear and concise perspective on the company and its prospects.
Please visit the forums tab for general discussion.
Just got off the PainChek webinar. I’ve neglected to report back to Strawman much on PainChek because I wasn’t sure what to make of them given slow (but steady) sales and a delayed FDA clearance. I got a bit wrong footed and exited just before a price run, but got back in in a dip and now have a small 1% holding and a ‘Lesson learned’ stamp in my growing ledger. There have been a couple of price spikes around FDA clearance excitement, but I suspect now is the grind towards operational breakeven (see below).
PainCheck now have FDA De Novo clearance for the adult product to sell into aged care, hospitals and care homes. They have one or two US customers - one pro-active customer had been waiting for several years for the FDA clearance. Key sales exec has moved his family to North America. US sales will not be farmed out, but there are software integration deals along with introductions from key software partners such as PointClickCare who cover 100,000 beds.
Meanwhile, sales in the UK and Australia are slow and steady (16% ARR and 18% contracted ARR growth). They have now tightened their contract integration times and require a 25% upfront payment. Due to growing market acceptance, contractual power and software integration is getting easier and they are I suppose learning as they go. Product retention is still only 85%, this needs to go up, may be a software/process integration issue that favours big players and market acceptance may help, but I have to ask about that if it continues.

Infant app is just now available in Australia and they will beta-test their pricing and marketing, and monitoring of conversion rates. Too early to say. There is talk of partnerships with big-pharma who like the idea of a test-treat combination. Something to watch.
They are going for a 10-1 share consolidation, timed with the AGM because it’s the cheapest way of approving that. They will stay on the ASX, CEO experience at Cochlear tells him this works fine, US investors will be happy to invest here.
Zero debt. Cash on hand - A couple of quarters. Obviously a raise is coming, but who gets involved, they are not saying, and there is that consolidation coming in November which is to appeal the the US investor. Mentioned institutions are now interested.
Operational breakeven in Australia and UK can happen in a year but they will continue to invest. Operational breakeven in the USA would happen when they have 10-15% market share. That would imply $15m US annual revenue if we are talking about aged care ($150m TAM).
US TAM for adults they estimate to be $500m per annum, covering aged care, hospitals and home care. This would take years to play out.
My take: Everything in aged care moves slowly. But…compliance pressures, FDA clearance and software integration are tailwinds. A few big US operators adopting would move the dial and they have the biggest Las Vegas conference coming soon.
PainChek has productive final FDA De Novo clearance meeting.
https://hotcopper.com.au/threads/ann-painchek-has-productive-final-fda-de-novo-clearance-meeting.8612442/
Based on the meeting discussions, PainChek is now compiling additional information collected from the completed clinical study that addresses the FDA feedback and aligns with the agreed actions concluded during the meeting.
This submission is expected to be the final step in PainChek’s US De Novo marketing clearance application and will be submitted prior to the end of June 2025. On receipt of the final PainChek submission, there is a commitment by FDA for a final decision on De Novo regulatory clearance within 75 days, giving a projected potential clearance date of mid-to-late September 2025 or sooner.
PCK. Not so Positive share Price reaction here though..
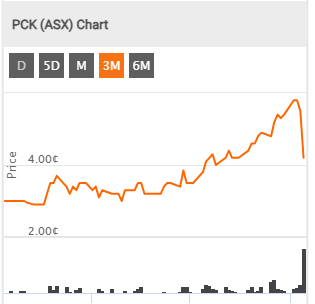
Last
4.2¢
Change
-0.013(23.6%)
Mkt cap
!
$77.36M

Not Held
Share price on a heater, in anticipation of FDA approval by July.
Meanwhile, contracted licence growth in Australia and the UK has been annemic. They seem to be fighting this battle one aged care home at a time.
They have very little cash left and will presumably wait for the hoped for good FDA news and then raise. Perhaps the cash situation is part of the reason sales and implementation has been so slow.
Infant phone ap is now being tested in the wild in Australia, getting feedback on pricing.
The USA sales will I think be assisted by PointClickCare, major US digital health pending partner.
Aged care rollout seems slow, as I feared. Still at 70,000 licences of 100,000 contracted. It seems like they've been referring to that 100,000 mark for a year now. They are working on improving the onboarding speed, there must be training bottlenecks.
85-90% retention. 50% have now renewed over 3 years.
News flow will be good this year, but there's still a need for more cash than they have:
- FDA clearance expected this half
- Infact app market testing and marketing plans underway, Aust launch by July
- Push into Aust and UK care homes and hospital markets (larger than aged care) but will the same training bottlenecks apply?
Not holding just watching.
Painchek has risen from 2.9c to 4.2c in the last week off no ASX announcements and with good trading volume. Investsmart (Alan Kohler) did interview the CEO, Philip Daffas on Thursday - perhaps that was the genesis? The next 4C is due within a week - so will know soon whether was a leaky ship or some are seeing some good long term value.
Some near term catalysts are:
- lodging of the FDA application for approval later this year
- hard launch of the Infant App in Australia. Supposedly launching internationally later this year
- Further growth of UK sales, which have been strong and now include partner channels
- Launch into Germany
- Update on trials - hospital, NHS and disability
In the investsmart interview, PD advised a capital raise likely to fund expansion into the US after FDA approval.
Own in SM and RL.
Raised $30m since backdoor listing in October 2016. Today market cap is $49.1m
· March 2024 Raised $5m, Placement $2.5m, $2.5m SPP at $0.0251 per Share
· September 2023 Raised $3.55m Placement at $0.027 per share
· June 2022 Raised $4.55m, Placement $3.0m, $1.555m Entitlement offer at $0.028 per share
· August 2020 Raised $10m Placement at $0.11 per share
· June 2019 Raised $4.15m Placement at $0.145 per share
· September 2017 Raised $2.75m Placement at $0.05 per share
· Backdoor listing October 2016
Quick check of Boards inside ownership, was less than 4% of total shares on issue.
I’m having a hard time figuring out if this pain assessment product would/should be sticky, and could ultimately become a part of standard care for non-verbal patients.
Retention
The promising early uptake I suspect has been somewhat supported by sentiment and government intervention (who can deny the sad plight of non-verbal pain sufferers and the tragedy of our aged care system) and the last quarter showed only 88% percent retention. The CEO explained away the low retention as ‘smaller providers who didn’t have the resources to properly integrate the system and we’ll go back to them’. Which makes sense but long term I imagine you need high 90s retention given that the pool of aged care centres and hospitals is not infinite.
The product
You can’t actually test the ap without an invitation. But I’ve watched a few videos of zoom call presentations by Painchek representatives to aged care staff. My first impressions are that it is simple to the point of simplistic. The facial scan registers micro-expressions of pain, and the ap also has as a formalised checklist of behaviours associated with pain. A checklist is useful to avoid subjective judgements about the patient but a checklist doesn’t require an ap - so the USP appears to ultimately be the facial scan.
Apparently it is all IP protected, which it better be because one of this year’s high powered AI models could probably eat Painchek’s lunch, as an entree. So, can they role out this product quickly and get it to become a part of standard care?
Is this really a $50 million dollar ap?
There is the a whole other potential source of revenue in the yet-to-be-released infant ap, but I’d imagine that either it goes viral or it doesn’t. It doesn’t lend itself to retention so it therefore require virality and the approval of family doctors.
Monday meeting
@Strawman , I feel that you have to be somewhat nice to your guests so they keep coming back, but I’d be happy to see Philip properly grilled on Monday. If this is a flash-in-the-pan I would love to know! I listened with alarm to your most recent Baby Giants discussion about ASX scams involving backdoor listings where Claude said ‘it has to be a WA company with a history in both mining and biotech’ and thought, oh crap, that’s what Painchek is! Philip is ex-Cochlear, so I can’t imagine he’d bother taking on a crap product to launch just before his retirement, but I don’t actually know him so that might be wishful thinking on my part.
After presumably getting coat-hangered by COVID, Painchek are back on their feet. Growing revenue, globally expanding, but not yet breakeven and still plenty of risks. They just did a raise.
Product: Their Aust and UK regulator-approved mobile app and system allows carers to scan the faces of non-verbal patients to detect expressions of pain. The app helps to distinguish distress caused by pain from other kinds of distress, to allow for more appropriate care and treatment decisions. They have data from 4 milllion pain assessments conducted on their platform.
Hurdles: Carers require some training in how to appropriately use the app and sales require deals with each aged care provider, and system integration, which I guess is a hurdle but perhaps also a moat-builder if they can become a part of regular workflow.
Strategy:
- They have a steadily growing presence in aged-care facilities in Australia (30% of market) and the UK (5%) with a focus on larger companies who can roll out the product across thousands of beds.
- The USA strategy is underway as described below under 'Near-term catalyst'
- They plan to expand to home-care which is 10x larger market than aged care.
- They plan to expand into hospitals, where dementia patients take up a meaningful proportion of beds.
- They have an infant app in the works to market to new parents - that will listen to the cry as well as scan the face.
Revenue: Their Australian and UK aged-care revenue has grown quarter by quarter for 60% ARR increase in a year and they claim 92% retention. They charge $50 'per bed' per annum. They are fast approaching 100,000 beds, which would cover operating costs but not expansion costs.
They just did a raise, dilluting by 10%, but reading between the lines they are attempting to time raises with an eye on milestones. USA has 2 million aged care beds.
I've jumped in early as a gamble, because the marketcap of sub $50 million seems reasonable if they can reach a small part of their addressable market. I'd be glad if someone more confident than me has anything to say about revenue verses market cap, I'm happy for a reality check. Thesis here is that the below catalyst will meaningfully lift the SP before any further raise, which the CEO basically alludes to if I heard him correctly.
Recent CEO presentation here: https://www.youtube.com/watch?v=mXo9DpekINs
Near-term catalyst: A validation trial is underway at two aged-care facilities in the US with the purpose of submitting results to the FDA for approval this year, maybe even this quarter. They already have 3 major partnerships in place with some aged-care providers covering half the market to begin selling once the approval is granted. CEO seems entirely confident that since the product is non-invasive and approved and in-use in Australia, the UK, and maybe EU, it should be approved by the FDA.
The infant app will also be launched this year in Australia. Considering $10 per month.
Leadership: I have little insight but CEO Phillip Daffas seems good on paper, was Global VP of marketing for Cochlear. He appears to be at the end of his career and this must be his swansong.
Ownership: I'm not sure what is a reliable source for insider-ownership data (if someone could please tell me) but Simply Wall St suggests 25%.
Competitors: Googling I could only find an iris-scanner startup which is privately funded, but that seems more invasive and would require more cooperation from the patient.
Discl: Own IRL and Strawman. Comments welcome as this is my first attempt to write an overview.
25-Jan-2021: Canaccord Genuity: PainChek (PCK): DecQ update - COVID-19 impact defers adoption
Analyst: Martyn Jacobs | Canaccord Genuity (Australia) Ltd. | [email protected] | +61 3 8688 9164
- Rating: BUY (unchanged)
- Price Target: A$0.35 (down from A$0.49)
- Current price: A$0.07
- 52-Week Range (A$): 0.06 - 0.20
- Avg Daily Vol (M): 3.7
- Market Cap (A$M): 83.4
- Shares Out. (M): 1,126.8
- Dividend/Shr (A$): 0.00
- Dividend Yield (%): 0.0
- Enterprise Value (A$M): 71.0
- Last Cash Balance (A$): 12.4
- Last Quarter Cash Burn (A$M): (1.4)
--- click on the link above for the full CG report on PCK ---
[I do not hold PCK shares.]
PainChek Limited (PCK) has developed a patented technology in the form of a mobile app that uses existing smartphone and tablet hardware, along with AI technology, to analyse facial expressions that indicate pain in real time. The technology can help carers identify the presence of pain when it isn’t obvious, quantify the severity of pain when it is, and monitor the effectiveness of interventions by aged care staff/medical personnel. Importantly, the device assesses pain criteria for accreditation and provides evidence to facilitate aged care operator funding. PCK’s Adult App has been approved by the Australian (TGA) and European (CE) regulators, and it will be seeking a De Novo clearance from the US FDA in CY20. While PCK is not yet profitable, we are attracted to the global opportunity, the traction in the Australian market, recent $5m Government grant and opening up of the UK market. These factors represent positive signs for this emerging operator that services a critical need. We initiate coverage on PainChek (PCK) with a BUY recommendation and a DCF based valuation of $0.55/share.
[Note: PCK closed at 21.5 cents on Friday (29-Nov-2019), so Canaccord's Martyn Jacobs is suggesting there's +155.8% upside in PCK - to reach his valuation of 55 cents.]
Target markets: PCK has two essential markets: those living with dementia and infants who suffer pain but cannot communicate it. Globally there are c.47.5m dementia sufferers, and the number of infants is c.400m. Importantly, the users of the device are the carers, health professionals, etc., and therefore the number of devices is actually multiples of the number of people experiencing pain.
Automating existing process in aged care: The device automates the existing Abbey Scale standard of pain assessment, which has been conducted manually. The data collected by the device can be seamlessly integrated into operator backend IT and administration systems, and these service providers represent a key distribution point for entering the aged care market. It is interesting to note and PCK advises that this is the first time a medical device has entered the aged care market before the hospital market. Note that the hospital and home care markets represent opportunities for PCK over time as well.
Infant's market entry expected FY21: The pre-verbal children’s application is currently undergoing a trial at the Murdoch Children’s Research Institute, with the study expected to be completed in 3QFY20.
Valuation: We use a two-stage DCF valuation and arrive at $0.55/share price target (WACC: 11.5%). While PCK is in its early commercialization phase, even revenue multiples don't do justice to an assessment of the potential for this business. It will clearly need to grow into its valuation to justify the premium in the share price. Yet we consider PCK is worthy of broad investor interest. This unique technology provides a breakthrough to an intractable problem in the aged care sector, which is in desperate need of technological solutions to support the care of the elderly and particularly those living with dementia.
Disclosure: Not held.