Did the market misunderstand the recent results, Im no expert in clinical trials but I do think it has been misunderstood...
The first question is what is a phase 2 trial.... simply to assess if there is a dosing efficacy to the compound (EFTI) + pembrolizumab (Keytruda) (in the TACTI-002) study. The answer is yes. They now have the data (and are approved) to progress to a phase 3 trial.
The data.
(TACTI-002) Assessing 2nd line non squamous head and neck cancer (2nd line is refractory to PD-1/PD-L1 treatment. which is a super important part to understand)
- Cancer patients (refractory to treatment) who had minimal physical disadvantage (could walk/ mobilise and had limited physical limitation). 37/39 participants evaluated with 30% of participants tumour progression free at 6 months.... That is a huge result given this was cancer non responding to other treatments.
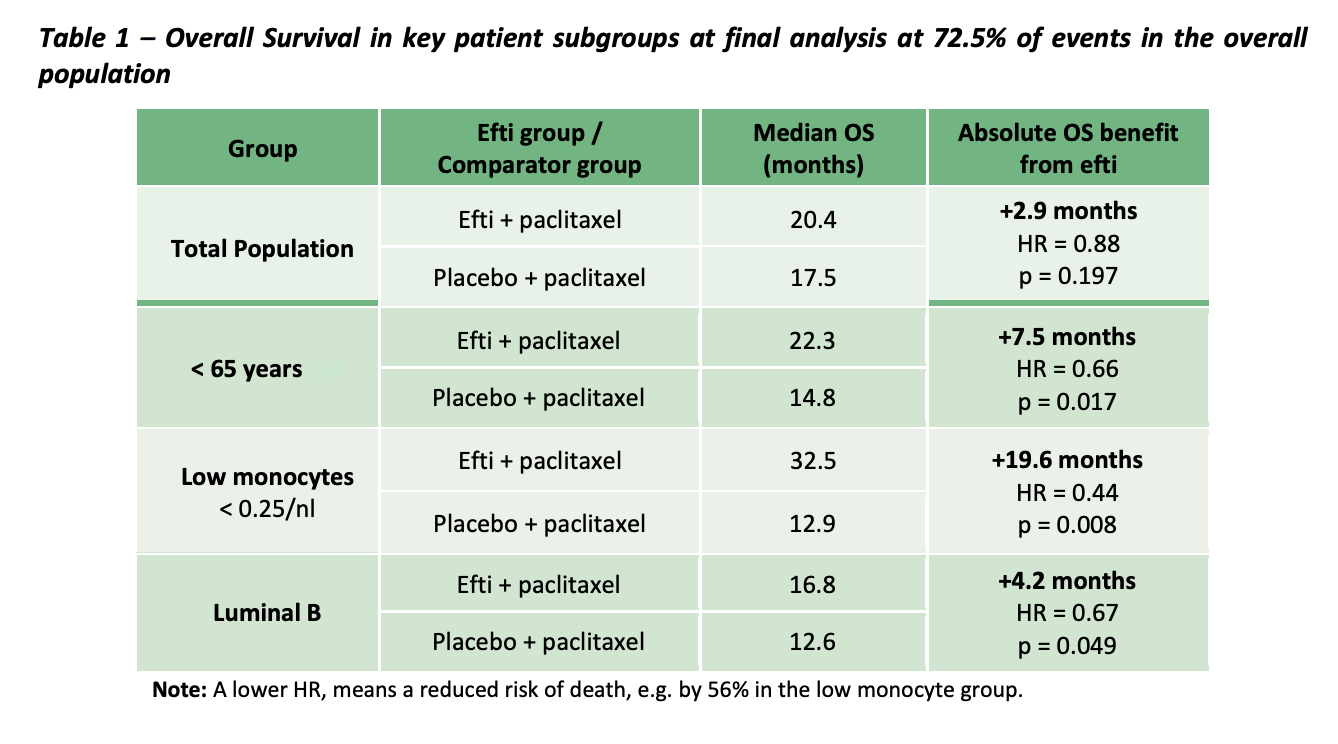
AIPAC (Active immunotherapy PAClitaxel + IMM's EFTI) is the largest and most progressed clinical trial, looking at hormone receptor positive metastatic breast cancers. It is important to understand how breast cancer treatment works. Essentially therapeutics target hormone receptors to slow/ destroy the tumour. Less hormone receptors = less treatable.... AKA Triple negative breast cancer is the most aggressive and least treatable breast CA.
- 114/227 participants (114 in the active group, the rest in usual care + placebo)
- In the overall study population EFTI + paclixatel showed a benefit of +2.9 months which doesn't seem that positive, however if the study population is further divided the results are much better.
- Patients who are younger (<65 had better results).. +7.5months survivability
- Patients with low monocytes had +19.6 months survivability (thats pretty huge)
- Patients with more aggressive 'luminal B' had a survival benefit of +4.2months.
A few points....
Clinical trials take time and there is still a phase 3 to go but overall I thought these were pretty good results, a phase 2 study is not designed to shoot the lights out, or in IMM's goal to cure cancer that is just simply not going to happen in this study but the definitely built a pretty impressive foundation for furthering this line of enquiry particularly in metastatic breast cancer. In terms of financials metastatic breast cancer is such a huge market with a huge burden of illness.
What didn't they report... lots of things. And my main question was what was the quality of life adjustment for these patients. For example the low monocyte group.... Did they 'live longer with really poor quality of life' or did this improve their ability to live and spend precious time with family.
Disclosure watching IRL and holding on strawman.
Mainly because I am a poor uni student who would back it given more appropriate circumstances.
Would really love feedback from some of the more medically educated people in here.



