Consensus community valuation
Straws are discrete research notes that relate to a particular aspect of the company. Grouped under #hashtags, they are ranked by votes.
A good Straw offers a clear and concise perspective on the company and its prospects.
Please visit the forums tab for general discussion.
On Friday 17th June 2022 the ASX only sent out a single free broker report as part of their free "ASX Equity Research Reports" service, and it was on Immutep (IMM) by Taylor Collison, so instead of posting this report link to the "ASX Equity Reports" forum here, I'm putting the link into this post under "Immutep".
To download the free broker report, click on the company name here: Immutep by Taylor Collison (Update). Or click on the file name at the bottom of this straw.
The report is dated 10th June 2022, so it's recent. TC rate IMM as an "Outperform", which I guess is a "Buy". But read the report in full and remember that sometimes brokers have reasons for being bullish on a particular company. For example, they might have raised capital for them, including providing placement services, or are positioning to do so in the future. Or they might have been involved in the float (the IPO) and have clients positioned in the company already. I haven't bothered to check if any of that applies to Taylor Collison with regard to Immutep, but as a general warning always take the bullish reports with a grain of salt and DYOR. I do not hold IMM shares myself and I do not follow the company.
Did the market misunderstand the recent results, Im no expert in clinical trials but I do think it has been misunderstood...
The first question is what is a phase 2 trial.... simply to assess if there is a dosing efficacy to the compound (EFTI) + pembrolizumab (Keytruda) (in the TACTI-002) study. The answer is yes. They now have the data (and are approved) to progress to a phase 3 trial.
The data.
(TACTI-002) Assessing 2nd line non squamous head and neck cancer (2nd line is refractory to PD-1/PD-L1 treatment. which is a super important part to understand)
- Cancer patients (refractory to treatment) who had minimal physical disadvantage (could walk/ mobilise and had limited physical limitation). 37/39 participants evaluated with 30% of participants tumour progression free at 6 months.... That is a huge result given this was cancer non responding to other treatments.
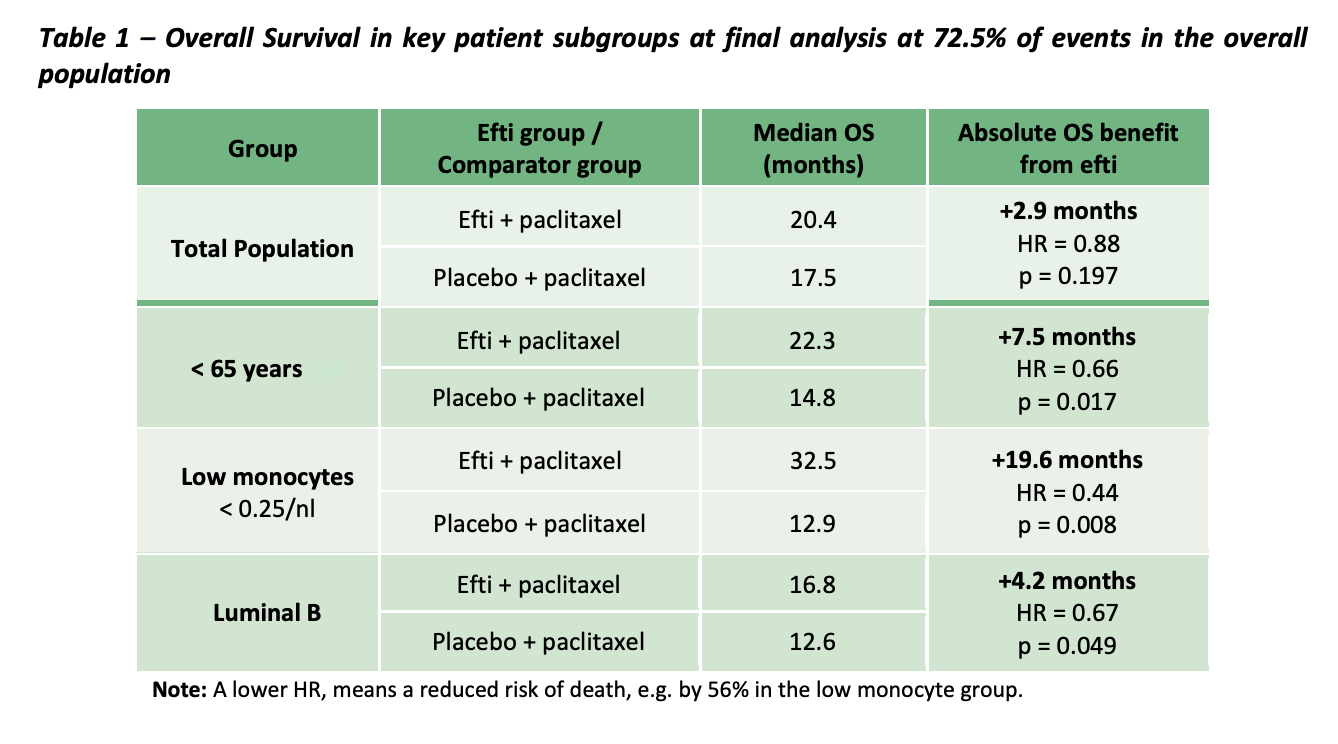
AIPAC (Active immunotherapy PAClitaxel + IMM's EFTI) is the largest and most progressed clinical trial, looking at hormone receptor positive metastatic breast cancers. It is important to understand how breast cancer treatment works. Essentially therapeutics target hormone receptors to slow/ destroy the tumour. Less hormone receptors = less treatable.... AKA Triple negative breast cancer is the most aggressive and least treatable breast CA.
- 114/227 participants (114 in the active group, the rest in usual care + placebo)
- In the overall study population EFTI + paclixatel showed a benefit of +2.9 months which doesn't seem that positive, however if the study population is further divided the results are much better.
- Patients who are younger (<65 had better results).. +7.5months survivability
- Patients with low monocytes had +19.6 months survivability (thats pretty huge)
- Patients with more aggressive 'luminal B' had a survival benefit of +4.2months.
A few points....
Clinical trials take time and there is still a phase 3 to go but overall I thought these were pretty good results, a phase 2 study is not designed to shoot the lights out, or in IMM's goal to cure cancer that is just simply not going to happen in this study but the definitely built a pretty impressive foundation for furthering this line of enquiry particularly in metastatic breast cancer. In terms of financials metastatic breast cancer is such a huge market with a huge burden of illness.
What didn't they report... lots of things. And my main question was what was the quality of life adjustment for these patients. For example the low monocyte group.... Did they 'live longer with really poor quality of life' or did this improve their ability to live and spend precious time with family.
Disclosure watching IRL and holding on strawman.
Mainly because I am a poor uni student who would back it given more appropriate circumstances.
Would really love feedback from some of the more medically educated people in here.
12-Nov-2020: Taylor Collison: Immutep (IMM): TACTI-002 update at SITC
Analyst: Dr DENNIS HULME, [email protected], +612 9377 1500, www.taylorcollison.com.au
Our View
IMM presented initial data from new TACTI-002 cohorts at SITC this week. On the positive side of the ledger, the continued high overall response rate (ORR) to efti/Keytruda combo therapy in the new head and neck cancer cohort (Part C) provides a very strong basis for a potentially pivotal randomised study in this indication. On the other hand, the ORR in the new cohort of first line lung cancer patients (Part A) was less than half that previously reported for Stage 1; subjects in the new cohort were older and in poorer health, which may have contributed to the lower ORR. When combined with the very high response rate in Stage 1 the ORR in Part A as a whole is ~50% higher than that previously reported for Keytruda monotherapy. The ORR in PD1/L1 refractory NSCLC was 4.4% in a challenging population. Overall, we believe the high response rates for efti combo therapy in the TACTI-002, TACTI-mel and INSIGHT trials should be sufficient to attract a substantial licence deal for efti. We expect the company to prepare for a randomised Phase IIb in head and neck cancer that aims to conclusively demonstrate that efti increases response rates when combined with checkpoint inhibitors such as Keytruda. IMM’s value is further supported by Phase II LAG-3 programs out-licensed to Novartis and GSK. We increase our valuation to $447m (vs $414m), $0.66/sh fully diluted (vs $0.63/sh) or $0.92/sh undiluted, based on the improved prospects in head and neck cancer.
Key Points
- TACTI-002 Part C: Positive new data in 2 nd line head and neck cancer – Initial data were reported for the first 10 subjects in the second cohort of patients (Stage 2) with squamous cell carcinoma of the head and neck (HNSCC) – 3 were not evaluable for tumour responses. There were 3 responders among the 7 evaluable subjects, including one complete response. The 43% ORR among evaluable patients was in line with the 44% (7/16) ORR among evaluable subjects in Stage 1. On an intention to treat (ITT) basis, including the nonevaluable subjects, the ORR was 36% for the 2 cohorts combined (including 3 complete responses), which is more than double the ORR of 14-18% reported for Keytruda monotherapy in comparable patient populations in the Keynote-012 and Keynote-040 studies. In our view these data are very positive and justify progressing to a randomised study of efti/ checkpoint inhibitor combo therapy in HNSCC patients. Stage 2 has recruited 17 of the target of 19 subjects. We expect data on the final 7 subjects in Part C to be reported in H121.
- TACTI-002 Part A: First line non small cell lung cancer (NSCLC) – IMM also reported initial results from the 19 subjects in second cohort of first line NSCLC patients (Part A). There were 4 tumour responses among the 16 evaluable subjects, including a complete response in a patient with 0% PD-L1 expression. The 25% (4/16) ORR among evaluable subjects is half the 53% (9/17) ORR reported for Stage 1. IMM noted that the subjects in Stage 2 were on average 9 years older (74 vs 65) and were in poorer health (84% vs 29% ECOG 1) than those in Stage 1. The 36% ORR for both cohorts combined on an ITT basis (including 2 complete responses) and 39% among evaluable subjects was ~50% higher than the 25% ORR to Keytruda monotherapy in Keynote-001. Responses were reported from all PD-L1 subgroups: notably, the ORR in low PD-L1 expressing patients (PD-L1<50%) was 31.6%, which is 68% higher than the 18.8% in comparable patients receiving Keytruda alone in Keynote-001. The efti/Keytruda combo was well tolerated with no new safety signals.
- Revised valuation assumptions: We increase the probability of approval of efti in HNSCC to 20% (vs 15%), bring forward a potential launch in HNSCC to 2026 and delay other efti launches to 2028. Our valuation increases to $447m.
--- click on the link at the top for the full Taylor Collison report on IMM ---