I believe the opportunity for Rhythm is huge, if they can execute a commercial strategy well.
Currently the most common procedure is at home faecal tests, which detect blood in the faeces. These are prone to false positives and not suitable for IBS or haemorrhoid sufferers. If a postive result the patient is sent for a colonoscopy, which can be extremely expensive. ColoSTAT accuracy & specificity has tested better than the standard currently used faecal tests.
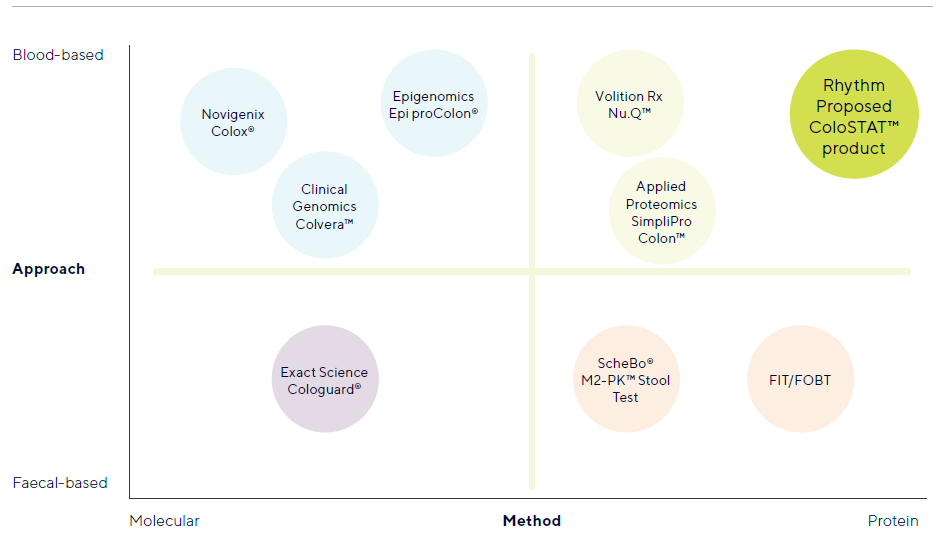
The company claims ColoSTATs main benefits are less invasive, more accurate, more cost effective and reducing the incidents of false positives.
In response to @GavCo concerns. The company aims to sell directly to pathology testing labs, and marketing the availability of a blood test to Doctors, who in turn can recommend ColoSTAT in lieu of their regular at home faecal testing.
Regarding competition. There are alternatives on the market or in clinical trials currently. ColoSTAT test will be used in standard lab testing where protein biomarkers are detected. Volution Rx Nu.Q is a similar solution which is still in trial phase. Applied Proteomics Simpli Pro Colon is another, which I cant seem to find much information on, perhaps someone knows whether this is on market and the effectiveness?
The other blood based tests on or entering the market are molecular tests which require specialist instruments and analysis. They also require extraction of DNA/RNA prior to testing. This can all be very expensive in comparison.