PainChek has productive final FDA De Novo clearance meeting.
https://hotcopper.com.au/threads/ann-painchek-has-productive-final-fda-de-novo-clearance-meeting.8612442/
Based on the meeting discussions, PainChek is now compiling additional information collected from the completed clinical study that addresses the FDA feedback and aligns with the agreed actions concluded during the meeting.
This submission is expected to be the final step in PainChek’s US De Novo marketing clearance application and will be submitted prior to the end of June 2025. On receipt of the final PainChek submission, there is a commitment by FDA for a final decision on De Novo regulatory clearance within 75 days, giving a projected potential clearance date of mid-to-late September 2025 or sooner.
PCK. Not so Positive share Price reaction here though..
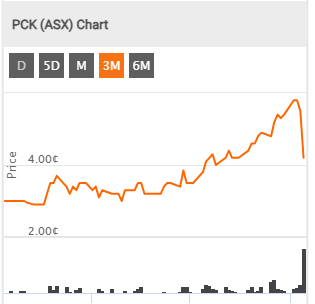
Last
4.2¢
Change
-0.013(23.6%)
Mkt cap
!
$77.36M

Not Held




