Price History
Premium Content
Premium Content
Premium Content
Straws are discrete research notes that relate to a particular aspect of the company. Grouped under #hashtags, they are ranked by votes.
A good Straw offers a clear and concise perspective on the company and its prospects.
Please visit the forums tab for general discussion.
- Recent
- Votes
Today I've undertaken a quick deep-dive (if there is such a thing) into Friday's announcement from $PNV on the application of Novosorb BTM in the treatment of diabetic food wounds (DFW).
This is a readout of a quick, initial scan to understand how excited I should be about findings.
TLDR: there is something here to consider, but I'm not in a rush to act.
I've investigated the following questions.
- How significant is the clinical result?
- How large is the market opportunity (starting with the US)?
- What other treatments are already serving this market?
- How might $PNV access the opportunity in the US?
- What's my overall view / implications for value?
1. How significant is the clinical result?
The acceleration in the time for the wound to heal is very significant: 191 days for BTM vs. 319 days for the SoC.
The ultimate improvement in outcomes is not statistically significant: 12 month healing rate of 66.7%for BTM+SoC vs. 56.5% for SoC but only with a p=0.48. Which means the results are barely distinguishable. i.e., a failed endpoint, were this a registration trial.
There was no significant difference observed in 12-month amputation rates.
So this looks promising, but it is important to understand that while the SoC is negative pressure wound therapy (NPWT) only, clinical practice in the US is already using a large number of alternative dermal substitutes with NPWT. So while the trial looks promising against the SoC, the industry has already largely moved beyond the SoC.
And here's the problem: I can find little if any evidence of clinical trials for these wounds pitting SoC alone against SoC + ny other dermal substitutes.
If fact, the whole field of how the various treatments have achieved their registrations and reimbursement codes is somewhat of a mystery to me and require further investigation. But that's for another day.
2. How large is the market opportunity (starting with the US)?
The overall DFW market is huge, and the segment of interest being post-surgical diabetes related neuropathic/neuroischimc wounds is anywhere from US$1.0 - US$2,5bn per year.
Importantly, the proportion of this market attributable to dermal substitutes appears to be in the order of US$0.3bn to $1.0bn p.a. (Sorry for the wide range, but the calculation combines varied factors, ranges and estimates)
3. What treatments are already serving this market?
In short, a lot. And more than I was expecting when I started the research.
I have (with support from my trusty BA) identifed no fewer than 23 existing dermal substitutes with relevant reimbursement codes, using a wide range of technologies including Novosorb BTM.
What? Novosorb BTM is already on the list? The answer is yes. And the reason is that many of the products appear to be being used in DFW using a more general registration code, and not exclusively a DFW code (or codes, as there are several procedures). That explains why Novosorb BTM is already being used, as it is being used under its more general registration for complex wounds.
How can this be, Well, some of the Novosorb super-users are general surgeons or trauma specialists, and they do sometimes treat DFW. So it is natural that they would give it a go. This is also consistent with the legion stories we've heard from David and recently departed Swami about "surgeon-led innovation".
The point is, it currently depends on surgeon initiative. $PNV reps. can't go in and say "Use this", "here's the evidence", "here's the reimbursement code", "order here". The trial is a step to changing that.
4. How might $PNV access the opportunity in the US?
It seems that much or even most of the DFW treatment is performed in the US by specialist Podiatric Surgeons. (DW has spoken about this repeatedly over the years).
The bad news for $PNV is that I think the burns, trauma and general surgeons in the locations which are BTM super-users perform a relatively small proportion of DFW treatment. Indeed, a large amount of the care is in outpatient settings. (Again DW has spoken about this before.)
I imagine (I don't know) that this means there will be only a limited overlap with the existing relationships and accounts for the salesforce. Resources will have to be reallocated or added, and new relationships built.
But importantly, many of these HCPs will have their existing preferred treatment methods, and will rely both on clinical evidence and economics to switch.
Now DW's most recent messaging on the costs of treatment starts to make sense. BTM is a relatively cheap product compared with many dermal substitutes, and has extremely high gross margins.
Therefore, I think if they can get the clinical evidence lined up, they could mount an assault on this market. But it will take condierable effort and time.
5. What's my overall view / implications for value?
I don't think attacking the DFW market in the US is going to be a quick process for $PNV.
By the looks of it, while the time-to-heal measure is good comapred with SoC, we don't know how it compares with other dermal substitutes. So it is not clear to me how $PNV can persuade a rusted-on podiatric surgeon to switch from their current practice.
The sales force will have to be augmented or retooled to go after this segment. That won't be quick.
The space already appears to be crowded with a vast array of choices for surgeons.
If you pushed me, I'd say that over 5-7 years, they might claw their way to a 10% market share in this segment, which could be anywhere from $US30 to US$100m incremental sales, as a BULL CASE.
That compares with FY25 US BTM sales of around US$57m sales, so it is a material opportunity and it has the potential to extend the growth runway in the US market.
Final Comments
These are very rough, back of fag packet calculations. For example, if existing practices are locked in, accessing practitioners is difficult, and the clinical data is judged not to be compelling or significantly differentiated to what the surgeons think they are already achieving, then maybe $PNV fights to get 5% or goes harder on price to get a bigger share. In that case it might not amount to very much ... maybe $US15m to US$50m p.a. incremental revenue in 5-7 years.
Alternatively, if the clinical data is built on over time and is strong, and it plays into a weak comparison set, and if US opinion leaders embrace the treatment, and if $PNV executes an effective sales strategy (perhaps partnering with a leading distributor in the podiatric market), them maybe they can get a bigger share, faster, pushing closer to US$100m p.a. in 5-7 years.
But there are a lot of "ifs" to get to that number.
It is impossible for me to be more definitive from this quick look. But I think I have sketched some bookends to think about. For sure, this looks interesting and seems to be an opportunity that DW and his team will be applying themselves to.
But I think there are a lot of both clincal and execution questions, so based on what I've learned, I'm not getting overly excited about it, just yet. It is clear that this is a competitive market with many treatment alternatives. Many more than I realised at the start of today.
Keen on the views of others who follow this sector.
Disc: Not held (yet)
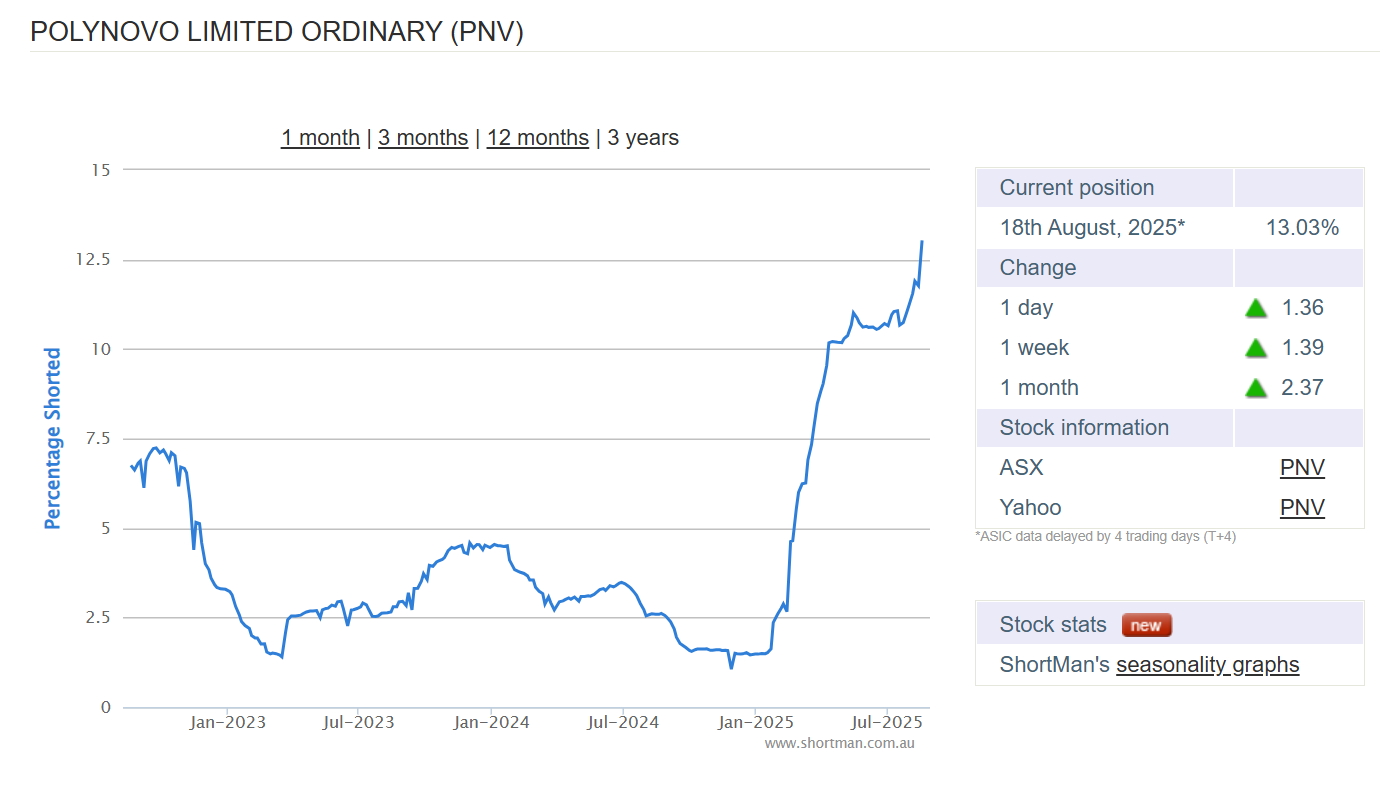
With the FY25 results for $PNV due this afternoon, it is interesting to note the short position (below).
3-year high - while the SP languished at $1.10.
Could be interesting.
Disc: Not held
$PNV's requested a halt as it is about to announce a "significant collaboration" with beta-cell technologies in Europe.
I'm assuming this could be a drug elution product that would use Novosorb as some kind of implant/drug elution carrier to use in concert with one of Betacells drugs? Drug elution technologies were always in prospect at $PNV, but we haven't heard much about it for a while.
Despite the trading haly, I am assuming that this would have to be proven through a full clinical trial program. My assumption is that it is early stage. But I am only guessing here.
There's nothing I can see on their latest pipeline chart (below from Macquarie Conference)

Very odd announcement, entitled a repsonse to an article in The Australian, rather than what it appears to be .... CEO Swami's intended resignation, at the request of the Board (DW?).
Very odd indeed.
The language makes it clear that agreement on the separation has not yet been reached. So is the announcement a device to force the end?
As some of us have speculated here, given the rapid fall in growth rate including the lack lustre progress in India, it was looking increasingly likely that Swami's incentives are likely out of the money, and that his retention was also a potential issue. Now it looks like that rather than jumping, he is being pushed.
Margin call gave a different lens on this issue, focusing on the behaviour of Chairman David Williams.
Whatever the reality and the truth, it appears all is not well with management and the Board at $PNV.
Disc: Not held
$PNV published their HY result, with the call starting in 3 minutes.
A few quick observations from me, not going over old ground that we've covered here before:
- Expense control OK, with Opex up +15.4% cf. Revenue +22.8%
- Operating Cashflow strongly negative .... why is this?
- Modest NPAT increase up to $3.338m compared with $2.694m in PCP
- New products still at the pre-clinical phase, with R&D spending modest
- New facilities on track.
Overall impression - nothing here causing me regret on my exit decision. I still think its trajectory has slowed too quickly for this to represent a compelling investment proposition. I don't have a view on fair value based on these numbers. But my model is shot, as is my thesis.
Here's a complete summary courtesy of my BA ChatGPT (I've had a quick look - it seems OK, but I've not vetted it in detail, so beware!)
PolyNovo 1H FY25 Performance Summary (Compared to 1H FY24)
Summary
PolyNovo delivered a strong first-half FY25 performance, marked by record sales, improved profitability, and strategic advancements in product development and market expansion. While operating cash flow turned negative due to increased investment in growth, the company remains well-funded, with A$30.5 million in cash reserves. With continued investments in manufacturing, R&D, and clinical trials, PolyNovo is positioning itself for sustained long-term growth.
Financial Highlights
- Total Sales: A$54.1 million, up 28.1% from A$42.2 million in 1H FY24.
- Total Revenue (Including BARDA): A$59.9 million, up 22.8% from A$48.8 million.
- Net Profit After Tax (NPAT): A$3.3 million, an increase of 23.9% from A$2.7 million.
- U.S. Sales: A$41.2 million, up 27.9% from A$32.2 million.
- Rest of the World (ROW) Sales: A$12.9 million, up 28.6% from A$10.0 million.
Cash Flow Highlights
- Net Operating Cash Flow: -A$12.5 million, compared to A$0.6 million positive in 1H FY24.
- This reflects increased payments to suppliers and employees (A$63.9 million vs. A$45.3 million in 1H FY24).
- Receipts from customers: A$44.3 million, up from A$41.1 million.
- Receipts from BARDA reimbursements: A$7.3 million, up from A$5.1 million.
- Net Investing Cash Flow: -A$4.5 million, compared to -A$0.5 million in 1H FY24.
- Driven by increased capital expenditure of A$5.1 million (up 361.2% from A$1.1 million) for R&D and new facilities.
- Net Financing Cash Flow: -A$2.2 million, compared to -A$1.4 million in 1H FY24.
- Repayment of borrowings (A$1.9 million) and lease liabilities.
- Cash & Cash Equivalents (End of Period): A$30.5 million, down from A$45.9 million at June 2024.
- Key Driver: Increased investments in infrastructure, R&D, and sales expansion.
Operational and Strategic Developments
- NovoSorb MTX Launch: Achieved A$2.1 million in sales following a successful U.S. launch in Q4 2024.
- Pipeline Expansion: Introduced new sizes and thicknesses for NovoSorb BTM and NovoSorb MTX.
- Hernia Repair & Plastic Mesh Development: Pre-clinical stage initiated for new medical applications.
- Full-Thickness Burns Clinical Trial (BARDA Supported): Achieved enrollment of 120 patients, paving the way for FDA engagement on potential paediatric burn treatment.
- Manufacturing Expansion: Construction commenced for a new facility in Port Melbourne (Operational by December 2025).
- Innovation Centre: Finalised design, with opening planned for June 2025.
Key Financial Metrics
- BARDA Revenue: A$5.4 million, up 10.2% from A$4.9 million.
- Capital Expenditure: A$5.1 million, up 361.2% from A$1.1 million, reflecting investment in R&D and infrastructure.
- Research & Development (R&D) Expenditure: A$5.1 million, up 3.8% from A$4.9 million.
- Employee Growth: Workforce increased to 282 employees, up 19.0% from 237 in 1H FY24.
Market & Growth Strategy
- Market Penetration: Focus on expanding U.S. market share, taking business from established competitors.
- Rest of World Growth: Increased procedure and market development efforts in Europe, the UK, and Ireland.
- Innovation & Expansion: Developing solutions for trauma, reconstructive surgery, and general surgical applications.
Disc: Not Held
Those following $PNV will no doubt be aware of the production problems competitor Integra ($IART) has faced following product recalls from the key Boston manufacturing facility. I got my BA (Perplexity.AI) to do a run down of the 4th November $IART analyst call.
Key message is that, while the problems have had a significant impact over the last year, $IART seems to be on the way back.
Clearly, in a market with a range of competing options in dermal repair, some surgeons facing supply problems will have been forced to switch. It will be interesting to see whether they switch back.
The next couple of quarters will likely be positively impacted by restocking orders, so over the coming quarters I'll need to analyse the Tissue segment sales across mutiple quarters to look through that.
It should also provide some insight into the overall progress of the segment, via comparisons with $AVH, $ARX and $PNV. While these companies have products that are not equivalent (there are both overlaps and complementarities!) a key question I am trying to get a better handle on is the respective growth contributions from 1) expansion of the market segement within established indications, 2) expansion into new indications, and 3) market share gains. My deep dive into this segment a couple of years ago (published here) made some assumptions about the market depth and breadth in the US. It's time to revisit that analysis, ahead of the learning about $PNV's US sales in February.
Here's the detailed analysis of the transcript
-------------
Integra provided several key points regarding its Tissue Technologies segment and recent production issues:
Tissue Technologies Performance
- Tissue Technologies sales were $110 million in Q3 2024, down 3.6% on a reported basis and 3.7% on an organic basis compared to the prior year.
- Excluding the prior year impact from the return of product manufactured in Boston, organic growth was down 9.4%
Production Challenges
- Production shortfalls for Integra Skin limited the company's ability to fully meet demand
- These production issues were the primary cause of the decline in Wound Reconstruction sales
Recovery and Outlook
- The company has been ramping up production of Integra Skin through the third quarter
- They expect to return to historical revenue run rates for Integra Skin in the fourth quarter of 2024
Other Tissue Segment Highlights
- Despite the Integra Skin challenges, there was strong growth across other parts of the Wound Reconstruction franchise:Low double-digit growth for DuraSorb
- Low double-digit growth for MicroMatrix and Cytal in the UBM platform
- Overall high single-digit growth in the UBM platform
- Private label sales were up approximately 13% compared to last year due to favorable order timing
International Impact
- International sales in Tissue Technologies were down mid-double digits, primarily due to the Integra Skin production shortages
The company appears to be addressing the production issues and expects improvement in the near future, particularly for Integra Skin products.
DW was interviewed this morning by John Hester, Bell Potter (Healthcare Analyst) at the Bell Potter Heathcare conference. No slides or significant information, apart from one. John took David head on about the lack of update or guidance at the recent AGM.
David explained that the Board is comfortable with analyst consensus, and if they weren't - either up or down - they would update the market. At the same time, he said they will not give forward guidance because, with their high growth, doing that is a pathway to trouble (I agree!)
So, the best view is that $PNV is on track to achieve about 28% revenue growth in FY25, with consensus at $134.5m. (My source: www. marketscreener.com. n= 9)
Disc: Held in RL and SM
Here are the Chairman and CEO speeches for the $PNV AGM to start in a few minutes.
NO trading update! (There should at least have been a 1Q revenue update, as they have an established record of updating even if irregularly - what are they playing at?)
Under their continuous disclosure obligations, if the sales information was materially different to what the market is expecting, then you'd have expected an announcement. So, at this stage I am assuming revenue is in-line.
Indeed the statement in Swami's speech "The US remains the driving force for our company, growing by 49% over prior year. As we expand into other trauma, infection and active complex wounds, the number of patients healed is expanding at a stronger pace."
"Expanding at a stronger pace" is definitely ambiguous, but it implies that the rate of sales growth is not slowing.
It will be interesting to see if there is an update after the meeting? There will certainly be Q&A on this topic!
(I cannot quiet the voice inside that says this level of obfuscation is deliberate.)
Disc: Held
Those who follow me here and also follow $PNV will know that I am NOT a technical investor.
However, I am always eager to learn, and so today plotted the chart below within my CommSec Ap. using the Bollinger band technicals views, which plots the Simple Moving Average (in this case 20-day) with the +/- 2 standard deviations. The basic idea being that when it flies above the upper limit, the stock is overbought and when is flies below, it is oversold. (I have no idea what other technical alogithms say ... @Saiton ... momentum traders are probably offloading?)
So, in this year's version of the $PNV rollercoaster, we've breached the lower Bollinger bound for the first time in over a year.
The other thing I'd note is that the volumes have been modest for a while now, in part because the shorts have quietened down quite a bit on this stock.
Let's overlay newsflow on that:
- 8th May - Record Month
- 23rd July - FY Trading Update (a good news story)
- 16th August - FY Presentation (confirm in the details of the good news story)
- 4th September - Pivotal Trial Complete (quite a good news story)
- 18th Sept and 2 Oct - Director Selling (not good news)
- Number of days since material good news: c. 95
Make no mistake, $PNV is still a highly-valued company, based on the fundamentals, and riding the rollercoaster is a condition of being a shareholder. But I think this is primed well for the next update.
So What?
I don't buy or sell on technicals, I only do that on valuation. And my current valuation is $2.60 ($2.30-$3.50). My problem is, that $PNV is already my largest RL holding. But it has fallen so far below my lower limit of valuation that it sure is tempting to take a small bite, particularly given that I am well within my maximum single stock exposure on a cost-base basis.
Decisions, decisions.
Disc: Held in RL and SM

$PNV have today announced the conclusion of the patient enrolment phase of the BARDA trial (PIVOTAL CLINICAL TRIAL), which started (I recall) in late 2021.
It has taken some 3 years to enrol 120 patients with full thickness burns (FTB) sufficient to meet the criteria, although the recent addition of India as an enrolment location has significantly accelerated the conclusion of the trial enrolment process.
It's not clear what the forward timelines are. Presumably, all patients have to progress through treatment to their end point. In the case of FTBs, I understand the primary end point is wound closure after 12 months. If it is required that all patients have to reach this end point, then there would be at least another year before submission of the data, and then around a further year for a Final FDA decision.
However, given that $PNV have repeatedly referred to ongoing dialgoue between BARDA, the FDA and $PNV, and given that many of the early patients will have passed the end point, as well as the repeated references to this in the last three investor presentations, I anticipate that the PMA decision may run to a shorter timeframe.
$PNV have been very disciplined not to say anything about timeframes (although DW always sails close to the wind on this), because ultimately, neither $PNV nor BARDA are in control of this. That said, given that BARDA is a government agency, then that probably counts for something.
Essentially, a FTB on-label indication will likely 1) lead to a BARDA stockpile purchase and 2) further accelerate adoption for FTBs in the US and 3) facilitate approval for FTB in those countries where FTB is not yet an approved indication. Note: it is used for FTB in many countries, and is already used for this off-label in the US.
Given all of this, the announcement is not price sensitive.
That, at least, is my understanding. Happy to be corrected by any StrawPeople who know better.
Disc: Held in RL and SM
Courtesy of DW's mail round, here are some of the analyst responses to the $PNV result:
Bell Potter: TP from $2.52 to $3.00; upgrade to BUY
Morgans: TP from $2.50 to $2.85; retained as ADD
Macquarie: TP from $2.75 to $2.85; retained as OUTPERFORM
Evans & Partners: TP $2.65; Positive
Wilsons: No TP - First Look only; OVERWEIGHT
OVERALL: Modest increases in TP overall. Generally positive
Given that DW has signalled the potential end of "record month" reporting, then 1H25 is the next catalyst as, in my view, the FY25 revenue growth assumption of the consensus is undemanding ($133.3 / $104.8 = +27%), which I expect will be readily surpassed even given a potential "BARDA" effect.
Disc: Held in RL and SM
Following the FY24 results, I have update my valuation for $PNV.
Result: $2.60 Range = ($2.30 - $3.50)
Method: 10 yr DCF
Key Assumptions:
- BTM/MTX only
- Market growing at 8% CAGR
- Assumes market share grows from 2.7% today to 15%/19%/22% by 2034 (Integra estimate of $2.5bn TAM burns & trauma)
- New products beyond BTM/MTX platform treated as a sensitivty to the Continuing Value Growth 5% instead of 4% base.
- %GM declines from 94% (2024) to 88% (2034) due to increasing mix of 1) developing markets, 2) complex product mix, and 3) large increments of capacity that will be under-utilised from time to time
- Capex to deliver just in time capacity: 1st $500m for $27m, 2nd $500m for $40m
- Range of expense/revenue ratios modelled from FY24 to reach reasonable benchmark for global medical device company by 2034.
- WACC 10%; Tax=30%
- Other minor assumptions for interest and working capital scale with revenue
Comments
Compared with my last detailed valuation from Sept-22, I consider the FY24 result puts a much firmer base under the low case.
@Parko5 my top end just hits your $3.50. This is worthy of a comment. There are plausible scenarios where I can get up to $4.00 without stretching my own belief, and so it's worth looking at what's not included (see section below). You'll also see that I contradicted myself a little about revenue growth in FY25. I do indeed model values ranging from 39% to 58%. The reason is a modelling convenience, as the market share increases linearly from today and I didn't break out BARDA. In practice, the "BARDA Effect" that I mentioned in the earlier post is a transient 1-year thing that hits 2025 disporportionately (I'm assuming). It has little consequence in the overall valuation, so I chose to ignore it, as my model is complex enough without adding more complexity!
What's Not Included
Upsides
1. BARDA full thickness burns approval by FDA in 2025, driving "higher for longer" growth rates
2. A strongly favourable IQVIA report in 2025/26 - showing economic and patient outcome advantages of Novosorb, leading to wide adoption by HMOs etc and accerlating market shift away from biologcs More "higher for longer".
3. Award of multiple Federal or State Government Tenders in India, leading to India rapidly taking off
4. Early entry to China beyond Hong Kong (pre-2027)
5. New Products (i.e., beyond BTM and MTX variants) commercialised before 2028 and achieving significant revenues before 2030.
Downsides
6. Emergence of alternative synthetics competitors eroding market share gains in the later years
7. Loss of momentum due to Integra-like product recall (These things happen in medical devices!)
1.& 2. can be argue to be contained within the high revenue growth assumptions. However, the impact could be more material increasing the 2034 market share by accelerating switching from competing treatments. They would also likley accelerate global adoption, particularly in countries without capacity to conduct their own trials.
Model Outputs and Inputs
(I can answer and provide more detail on methods used to get to each of the items below, if anyone wants.)
1. Valuation
 2. Model Output Table
2. Model Output Table

3. Input Tables

"i" related to FY24; "f" related to FY34 - model shows linead trend in expense ratios across 2024-2034

Disclaimer: This is intended for my personal use only. It is not advice and must not be used as the basis of an investment decision.
I'm limbering up for the DW Show at 2pm this afternoon with a quick review of the $PNV results. The financials have been well-telegraphed in advance, and I've gone through the Accounts and it's all remarkably close to my forecasts.
FY24 Results and Financial Statements

Their Highlights
- Total revenue including BARDA of A$104.8m, up 57.5% on STLY of A$66.5m
- Strong growth in U.S. achieving record sales of A$68.7m up 49.0% on STLY of A$46.1m.
- ROW sales of A$23.4m up by 73.3% on STLY of A$13.5m.
- Positive cash flow from operations of A$3.7m up 155.7% on STLY (A$6.6m)
- Net profit after tax of A$5.3m (FY23: A$4.9m loss)
- At year end the business had A$45.9m cash and cash equivalents
During the Period, the Company’s other key initiatives and achievements include:
- Record monthly sales in April of A$9.2m (monthly revenue: A$10.5m) and May of A$9.8m (monthly revenue: A$11.3m).
- Initiated a full market launch campaign in the U.S. for NovoSorb MTX in June 2024.
- Strengthened the U.S. team from 93 to 107 (June 2024)
- Increased U.S. customer accounts by 197 from 299 to 496 (June 2024)
- Increased global employee headcount from 218 to 254
- New C-suite roles - Chief Medical Officer, Chief People Officer, General Counsel and President, Asia Pacific.
- Supplied into certain war zones for humanitarian needs.
- Obtained registrations for Bolivia, Ecuador, Thailand, and Sri Lanka.
- Enrolled 120 patients into the U.S. BARDA pivotal trial for full thickness burns.
- Finalised design and selected a builder of the third manufacturing facility to service up to $500m in additional revenue.
- Awarded Victorian Government grant of A$2 million for R&D facilities expansion, subject to customary conditions.
My Analysis
The key for today is that $PNV delivered on their commitment at the capital raise to be profiable in FY24.
EBITDA, EBIT, NPAT, and FCF all positive. The first year we've had this.
Gross Margin % of 94.8%
In the US, with modest sales team growth, new accounts and revenue grew strongly, reflecting the lag effect of 1-2 years between adding headcount and driving revenue per account. A key question is where is the US trajectory from here?
2024 has been a foundational year: 1) broading market approvals across the global where Novosorb can be sold 2) progressing the design of the major manufacturing capacity expansion, 3) re-igniting R&D to build out further products to exploit the platform technology (R&D expense in FY24 up to $11m from $7,4m, but still only a CSL-esque 10% of revenue), and 4) building out the management team. These are all important steps in building a business from this start-up with a genuous product.
In the Chairman and CEO remarks, there were further details on revenue progression in key markets:
- UKI up 81.5%
- EU/Germany distributor markets up 81.2%
- Australia up 38.7%
I take the UKI as a good indicator of what the EU can ultimately do, and it looks like the distributor is kicking into gear. UK/EU growth will be important in maintaining the group trajectory as the US inevitably matures.
The addition of licensing in SUPRATHEL means it looks like $PNV are taking a leaf out of the $AVH book. Once you have the sales foot print, who need to give them more things to help drive contribution margin per account. So, good.
What's not mentioned - India. There is a lack of granularity there. So hopefully the analysts will try to tease out some more on that on the call. After all, we've had 20+ people now working that market for over a year, and there have been some qualitative stories of progress. India does not need to be a "today thing", however, over the longer term the potential for the product to get traction at a reasonable contribution margin in middle income/developing markets helps the long-term growth thesis. (And after all, it's why - or one reason why - Swami joined the company!)
My Key Take Aways
Report entirely as expected. No surprises. So the question is what else can we learn on the call at 2pm.
Lunch now, and then I'll be sure to get my ringside seat for the DW show!
Disc: Held in RL and SM
$PNV is presenting at the Canaccord Genuity Growth Conference.
There's no market sensitive or significant news we haven't heard before, but we don't always get the information in the most cohesive manner (!!), so this might be of interest to some. The presentation pulls everything together quite nicely.
What has been interesting in the last two conference presentations, is that the portrayal of new products and timeframes has re-emerged, after an absence of over three years. The new head of R&D has been in place for over a year now, so this makes sense.
Looking forward to the FY results - not expecting any surprises or material new information, but hoping for a little more granularity on how some of the RoW markets are tracking. Also, I'm looking to see if there has been any signficant change on capex, not that the costs for the new facilities must be in.
With RoW at $23.3m sales and 73.1% annual growth, that's ahead of where all of $PNV was in 2020, when it was growing at 54%! Just reflect on that.
Disc: Held in RL and SM - my largest position in both
DW has just circulated two analyst notes to everyone on his email list. Macquarie (who are maintaining their TP of $2.75 and Outperform) and Wilson (who are at $2.65 are Overweight). Neither are updating recommendations and I assume are awaiting the FY, with its forward looking statements.
My reason for this Straw is that the Wilson report contains some interesting insights, both on the US and also on PMI the distributor in Europe - who is running a full thickness burns trials. The commentary is rather encouraging and succinct, so I've extracted the relevant paragraphs.
From Wilson's Report
"Polynovo has pre-released its unaudited revenue results for FY24. US sales increased 49% to $104.8M; with ROW sales up 73% to $23.3M. US performance in 2H24 was 8% lower than we forecast; offset by a 10% beat from ROW businesses plus grant support from BARDA. We’re not allowing a $3.5M sales ‘miss’ in USA dissuade us from our O/W thesis on PNV. Burns still constitutes ~68% of product volume and is invariably lumpy. Anecdotally, PNV was winning up to 70% market share by surface area in key US centres over 4Q24, thanks to its competitor’s protracted difficulties (Integra’s recall). We assess that >30% of the absolute US growth dollars in FY24 has come from pricing, with more to come, as described in our recent upgrade. The ROW business stands to benefit in FY25e as well, with several jurisdictions set to re-tender business"
"The absence of Integra’s PriMatrix and SurgiMend in the marketplace created a $30-40M annualised revenue opportunity in burns, trauma and reconstructive surgery. Notwithstanding hefty price increases, BTM remains super-competitive on (per cm2 ) pricing and enjoys a broad clinical following. Feedback suggests BTM has usurped Integra now on a volume basis (at least in some major ABA accredited burns centres) and carries that incremental share tailwind into FY25e. Internationally, we understand that PMI’s markets (distributing into Germany, Austria, Switzerland), Spain and Turkey starred. BARDA revenue support was also higher than forecast, given how quickly Polynovo’s PMA-directed trial in full-thickness burns has enrolled."
"Forecasts under review. At this stage we see little change to revenue forecasts, expecting continued market share gains (both organically in burns and at Integra’s expense), incremental volume via extension into trauma indications and flagged pricing momentum. Thinking about costs leading into FY25e, we’re cognisant of a few (positive) areas where investments may be drawn forward (e.g. SKU expansion for MTX, building on early product traction in trauma; and an earlier PMA filing in relation to full-thickness burns)."
Disc: Held in RL and SM
I'm not sure how much notice the market is paying to Broker/Analyst views for $PNV, however, I wanted to share some analysis which I know some of us will find interesting.
My valuation
First, I want to state that I won't be updating my own model until after the FY results are out. But for the record, my valuation is $2.37 (if I adjust my $2,25 from Feb-24 by a further manual adjustment of +5%)
My last full evaluation across a range of scenarios from almost a year ago was: $2.16 ($1.63 - $3.63), which if I roll forward by one year becomes $2.37 ($1.80 - $4.00). \
Now when I update the model in late August, I'll have to re-run the growth scenarios, have an updated cost structure and view on capex. So, it could end up looking quite different. My sense, however, is that the range will narrow, and that the low end of the range will come up.
For those of us who who might respond with "what good is a valuation with such a wide range?", my answer is that it represents unresolvable uncertainty. Remember, $PNV is still passing through the inflection point so, good luck justifying a tighter range.
Brokers Consensus
I use two sources when I look at brokers/analysts:
- Marketscreener.com: $2.18 ($1.00 - $2.75); n=8
- Tradingview.com: $2.46 ($2.00 - $2.75); n=5
I'm going to ditch the low value on marketscreener.com of $1.00, because I think it has no credibility. That moves the marketscreener.com average TP to $2.34 ... closer to Tradingview. (Note: on these services I can't see the individial datapoints)
Because of my concern in this case about the marketscreener.com dataset, I am going to focus the rest of the analysis on the analysts in tradingview.com.
So, based on today's close of $2.60, the market is now only about 6% ahead of the analysts.
Revenue Growth
The insight I wanted to share, is that over the next 6-9 months there is a chance for a material re-rating of $PNV.
I'll make the case by focusing on revenue growth - because it is still the dominant factor.
The analyst "consensus" for revenues are as follows, with % growth yoy in parentheses:
FY24: $104.8m (+57.5%)
FY25: $133.5m (+27%)
FY26: $167.8m (+26%)
Of course, it is reasonable to expect revenue growth to start tailing off at some point, but if you consider the last 3 annual y-o-y growth rates of FY21 (32.0% - COVID access impact) FY22(42.8%) FY23(58.8%) FY24 (57.5%), something dramatic would have to happen in FY25.
However, we know that:
- The category is expanding
- BTM is taking market share
- MTX is now also being rolled out
- The global rollout is still firing on all cylinders
My Conclusions
I'm not a seller of any $PNV much below about $3.80.
Of course, it will continue to be volatile. But the risk of getting off the bus and then being unable to get back on with this one is just too great for me.
I believe this is going to get more focus from the market as we move through the inflection point - i.e. in FY25. Of course, I realise that - for a couple of years at least - some "talking heads" will shake their heads at eye-watering P/Es. But that is an irrelvant measure AT THIS STAGE.
I'm very interested to see what upgrades come through for FY25 and FY26 over the coming 6-9 months, and everything that entails.
If I wasn't at a full allocation, I'd still be a BUY today.
Finally, I said in another straw recently, that I would test all valuations in healthcare through an M&A lens. I have not yet done that for $PNV. I will in August.
Disc: Held in RL and SM
Especially for you @Rick !
Their Headlines:
• Total revenue including BARDA of A$104.8m up 57.5% on STLY of A$66.5m.
• FY24 sales of A$92.0m up 54.5% on STLY of A$59.6m.
• Strong growth in U.S. sales of A$68.7m up 49.0% on STLY of A$46.1m.
• ROW sales of A$23.3m up by 73.1% on STLY of A$13.5m including strong performances in developed markets like UKI, Germany and ANZ.
• Surgeon education and charitable contributions, widely used to support patients in conflict zones.
My analysis
Revenue is bang on where I expected. I had them coming in anywhere between $102m and $106m, which was easy to pick, given the absence of "record months" in the last few months.
All this should be good enough for them to hit their positive NPAT commitment for the FY.
I'm pleased revenue growth is holding up strongly. Analyst views have this starting to decline in % terms quite quickly over the next two years and, if they can hold it around +50%, then that's the opportunity for the next significantly leg up in SP, as that will drive a few years of very high EPS growth.
Conclusion.
On track. Holding for long term. My biggest RL position.
Oh @Rick - ye of little faith! ;-)

Although enjoying volatility is part and parcel of being a long term $PNV holders and, in that context, the price action of recent days is simply par for the course, I found the announcement below in a search of the news-wires. It came out last week.
It is not surprising that $PNV didn't make an announcement, as in my view it doesn't have a direct or material bearing on $PNV's sales, but it is good that $PNV is being sought out by innovators for collaborations.
Full text follows.
---------------------------------------
Spectral AI Announces Collaboration with Global Wound Care Company PolyNovo to Introduce DeepView System for Burn Indication to Australian Market
861 words
8 July 2024
12:00 GMT
GlobeNewswire
PZON
English
© Copyright 2024 GlobeNewswire, Inc. All Rights Reserved.
Spectral AI Announces Collaboration with Global Wound Care Company PolyNovo to Introduce DeepView System for Burn Indication to Australian Market
DALLAS, July 08, 2024 (GLOBE NEWSWIRE) -- Spectral AI, Inc. (Nasdaq: MDAI) ("Spectral AI" or the "Company"), an artificial intelligence (AI) company focused on medical diagnostics for faster and more accurate treatment decisions in wound care, today announced that it has signed a Memorandum of Understanding ("MOU") with global medical device company and burn wound therapy leader PolyNovo Limited ("PolyNovo") under which the companies will collaborate to assist Spectral AI in a potential limited deployment of its DeepView System for burn indication in Australia.
Under the MOU, PolyNovo will support Spectral AI's application to the Australian Special Access Scheme (SAS) with an ultimate goal of allowing Spectral AI to deploy up to two DeepView Systems at the Royal Adelaide Hospital and The Alfred Hospital in Melbourne to lay the groundwork for the Company's eventual commercial roll-out based on clinician evaluations and experiences.
The SAS was introduced by Australia's Therapeutics Goods Administration in recognition that there are circumstances where patients need access to certain medicines, medical devices, or biologics that are not already included in the Australian Register of Goods.
Spectral AI's DeepView(TM) System is a predictive device that offers clinicians an immediate and objective assessment of a burn wound's healing potential prior to treatment or other medical intervention. The image processing algorithm employed by the DeepView(TM) System utilizes multispectral imaging that is trained and tested against a proprietary database of more than 340 billion clinically validated data points. The DeepView(TM) System is non-invasive and cart-based, allowing for exceptional mobility within the healthcare setting.
PolyNovo develops and sells patented, bioabsorbable, synthetic, polymer technology used to reconstruct complex wounds, including deep dermal and full--thickness burns, and aid the body in generating new tissue. PolyNovo's FDA-approved NovoSorb(R) BTM (Biodegradable Temporising Matrix) and NovoSorb(R) MTX product portfolio is available in 37 countries around the world.
"PolyNovo's innovative therapies have proven to be life changing and it is one of the world's most respected providers of burn treatment solutions," said Peter M. Carslon, Chief Executive Officer of Spectral AI. "Understanding when it is appropriate to apply these therapies is paramount to realizing improved patient outcomes. We believe that the Day One wound healing assessment provided by the DeepView(TM) System empowers clinicians with the knowledge to make an informed and rapid diagnosis when time is of the essence. We are honored to work with an established market leader as we take these initial steps to familiarize clinicians in Australia with Spectral AI's technology, support their life-saving work, and help to elevate the level of patient care."
About Spectral AI
Spectral AI, Inc. is a Dallas-based predictive AI company focused on medical diagnostics for faster and more accurate treatment decisions in wound care, with initial applications involving patients with burns and diabetic foot ulcers. The Company is working to revolutionize the management of wound care by "Seeing the Unknown(R) " with its DeepView System. The DeepView System is a predictive device that offers clinicians an objective and immediate assessment of a wound's healing potential prior to treatment or other medical intervention. With algorithm-driven results and a goal to change the current standard of care, the DeepView System is expected to provide faster and more accurate treatment insight towards value care by improving patient outcomes and reducing healthcare costs. For more information about the DeepView System, visit www.spectral-ai.com.
Forward Looking Statements
Certain statements made in this release are "forward looking statements" within the meaning of the "safe harbor" provisions of the United States Private Securities Litigation Reform Act of 1995, including statements regarding the Company's strategy, plans, objectives, initiatives and financial outlook. When used in this press release, the words "estimates, " "projected," "expects," "anticipates," "forecasts," "plans," "intends, " "believes," "seeks," "may," "will," "should," "future," "propose" and variations of these words or similar expressions (or the negative versions of such words or expressions) are intended to identify forward-looking statements.
These forward-looking statements are not guarantees of future performance, conditions or results, and involve a number of known and unknown risks, uncertainties, assumptions and other important factors, many of which are outside Company's control, that could cause actual results or outcomes to differ materially from those discussed in the forward-looking statements. As such, readers are cautioned not to place undue reliance on any forward-looking statements.
Investors should carefully consider the foregoing factors and the other risks and uncertainties described in the "Risk Factors" sections of the Company's filings with the SEC, including the Registration Statement and the other documents filed by the Company. These filings identify and address other important risks and uncertainties that could cause actual events and results to differ materially from those contained in the forward-looking statements.
Investors:
The Equity Group
Devin Sullivan
Managing Director
Conor Rodriguez
Analyst
Some interesting information in the Macquarie conference presentation.
Two items caught my eye:
- A picture of the capacity resulting from the investment in new facilities. (Not see before although elements of the information have been disclosed before).
I don't recall them before ever having said that existing facilities support a capacity of 180,000 devices (please correct me if I am wrong).
The linkage to revenue is a bit ambiguous, but on one reading it does indicate that in FY25 existing facilities will max out if revenue growth is significantly greater than consensus c. 30% - which I expect it to be. It would have been good to hear the Q&A in the meeting!

2. It's a while since we've seen references to new products - and this time with an indicative timeline. Frankly, I am surprised to see the short term timeframe for SynTrel and Syntrix. But then again, we know surgeons are already using MTX internally, so perhaps its not such a leap.
I wonder if this means they've cracked the polymer property issues which appeared to be holding them back when this was discussed at the AGM. Must be - otherwise the short term timeline is foolhardy, to say the least.
It will be interesting to hear the commenary around this slide at the FY results.

On today's call, DW is referring to a comparison study putting Novosorb head-to-head with Integra's animal-derived product. I think this might be it.
It looks like a big deal - particularly given the overall healthcare economics findings, which is going to be important to the payors, particularly the health funds in the US. However, healtcare economics may also influence guidance in nationalised systems like UK, and reimbursement systems like Medicare in Australia.
==============================
Comparative Analysis of Animal-Derived vs Fully Synthetic Acellular Dermal Matrices in Reconstructive Surgery An Examination of Clinical, Aesthetic, and Economic Measures Timothy Olsen, MBA, Safi Ali-Khan, MD, and Derek Bell, MD, Annals of Plastic Surgery, Volume 92, Supplement 2, April 2024
Introduction: The fully synthetic skin substitute, NovoSorb Biodegradable Temporizing Matrix (BTM), may be a cost-effective alternative to the animal-derived Integra Dermal Regeneration Template (IDRT). However, the current literature insufficiently compares the two. Therefore, our study compared clinical, aesthetic, and economic outcomes in treating soft tissue wounds with IDRT, an animal-derived template, vs BTM, a fully synthetic template
Methods: Our single-center retrospective study compared outcomes of 26 patient cases treated with BTM (57.7%) or IDRT (42.3%) during 2011–2022.
Results: The mean surgery time was significantly shorter in BTM cases (1.632 ± 0.571 hours) compared with IDRT cases (5.282 ± 5.102 hours, P = 0.011). Median postoperative hospital stay was notably shorter for BTM placement than IDRT placement (0.95 vs 6.60 days, P = 0.003). The median postoperative follow-up length approached a shorter duration in the BTM group (P = 0.054); however, median follow-up visits were significantly lower in the BTM group compared with the IDRT group (5 vs 14, P = 0.012). The median duration for complete wound closure was shorter for BTM (46.96 vs 118.91 days, P = 0.011). Biodegradable Temporizing Matrix demonstrated a notably lower infection rate (0.0%) compared with IDRT (36.4%, P = 0.022). Integra Dermal Regeneration Template exhibited higher wound hypertrophy rates (81.8%) than BTM (26.7%, P = 0.015). Revisionary surgeries were significantly more frequent in the BTM group ( P < 0.001). Failed closure, defined as requiring one or more attempts, exhibited a significant difference, with a higher risk in the IDRT group (26.7%) compared with BTM (6.7%, P = 0.003). Biodegradable Temporizing Matrix showed a lower mean Vancouver Scar Scale adjusted fraction (0.279) compared with IDRT (0.639, P < 0.001). Biodegradable Temporizing Matrix incurred lower costs compared with IDRT but displayed a lower mean profit per square centimeter ($10.63 vs $22.53, P < 0.001).
Conclusion: Economically, although the net profit per square centimeter of dermal template may favor IDRT, the ancillary benefits associated with BTM in terms of reduced hospital stay, shorter surgery times, fewer follow-up visits, and lower revisionary surgery rates contribute substantially to overall cost-effectiveness. Biodegradable Temporizing Matrix use reflects more efficient resource use and potential cost savings, aligning with broader trends in healthcare emphasizing value-based and patient-centered care
$PNV have announced the Indian Government has approved Novosorb BTM for inclusion on the e-Marketplace.
I'm not sure whether this, of itself, directly leads to material sales, as what is needed are the tenders in process to put in place the contractual frameworks that enable publicly-funded hospitals to make purchases.
Of course, $PNV are already selling into the private sector. As a 2020 there were 44k private hospitals (1.2 million beds) versus 26K public hospital (700k beds), so the private sector is very important.
While the release below indicates real traction in India, it would be nice to get some $ and margin insights in due course.
I'm not grumbling, though, this is good news and we will no doubt hear when the first public tender is awarded.
Full Text of the Announcement
The Company is excited to announce that the Indian Government has approved NovoSorb BTM to be included in the Government-e-Marketplace (GeM) portal.
GeM is a centralised procurement platform for Government hospitals that can now buy NovoSorb BTM throughout India. This approval provides access to supply BTM across all the Defence hospitals, Railways hospitals, ESIC (Employees State Insurance Corporation) hospitals and the various AIIMS (All India Institute of Medical Sciences) hospitals. We expect the first order within a month.
Simultaneously, our India team has been participating in several government tenders which will enable the Company to supply public hospitals.
Sales in private hospitals in India have been growing rapidly on a month-on-month basis.
PolyNovo participated at the National Academy of Burns of India Conference (NABICON) from 15 to 17 February 2024, the flagship event for burn surgeons. This was attended by 180 burn surgeons from all over India. In addition to a PolyNovo sponsored symposium, featuring U.K. surgeon Dr. Pratap Dutta, there were several other presentations featuring NovoSorb BTM by Indian and U.S. surgeons.
Chairman, David Williams said: “Our India team is optimistic we can win a number of other tenders that will open the doors of public hospitals for BTM.”
CEO, Swami Raote said: “It will be a huge step to be able to access burn patients admitted to public hospitals for treatment. NovoSorb BTM will enhance the standard of care and significantly improve the quality of life for Indian patients.”
This announcement has been authorized by PolyNovo Company Secretary Jan-Marcel Gielen.
Disc: Held in RL and SM
I've already shared some analysis on the $PNV financial results in earlier Straws, which I do not repeat here. However, I commented earlier this week that David, Swami and Jan provided a lot of detail both in their voiceover on the presentation and also in the Q&A.
So, I thought I'd share the key nuggets I extracted from going back over the call and the transcript. In total, I think it provides a much richer picture of the strength of this business, and underscores my bullishness on it as a long term growth stock.
I'll bear all this in mind when I do my major valuation update at FY. There is a lot to consider.
So here goes.
--------------------------
My Overall Key Takeaway: Sustained, capital efficient, long-term growth, driven by existing and new markets, existing and new indications, and potential new platforms. $PNV is now profitable and cash generative.
Key messages
- Strategy and capital allocation - long term growth
- Financials - outperformed their internal budgets and plans
- Capex - material step up in FY25 to build "Mega" for $25m, but fully budgeted in 2022 capital raise
- Markets - now serving 37 markets with increasingly material RoW growing strongly
- Organisation - now built out, so future growth in headcount slowing
- Clinical development - clinicians leading broad expansion of indications; potential for multiple new platforms
1. Strategy and Capital Allocation
DW made clear the strategy is to keep expanding “both in indications and in geographies” wherever they can see the margins. He said this in clarifying feedback he has received on his statement that he doesn’t care about profits. The clarification is that by focusing on growth where he can see margins, then profits will follow. He tweaked his rhetoric by saying, “if you want dividends, you’ll need to sell some shares.”
2. Financials
All details covered in previous straws, but CFO Jan made the comment that they have achieved profitability earlier than budgeted because sales have been above plan
For example, the $8m month in November wasn’t budgeted until April
On cashflow, the business is essentially cashflow breakeven.
DW discussed reporting. He said there has been feedback on their approach of reporting "record sales months". He's discussed it with the Board, and they've decided to continue because they want to keep investors informed as key milestones are achieved.
3. Capex
On the new facilities, the main investment is yet to come
1H24 Capex of $1,1m was design work for facilities plus some R&D equipment
2H Capex “marginal increase in capex as the design process nears completion and we expect to commence construction in 1Q FY25.”
Total planned capex for the third “Mega” production line is $25m over two-year period
Guidance on the spend profile will be given once design is completed (FT24?)
Until new facilities are ready there are no issues with current manufacturing output form the existing two lines, which see continuous improvements in output and efficiency, evidence by the very low % Gross Margin.
Mega will be designed to be modular and scalable, as they plan to have to accommodate many more SKUs than at present.
(My note: Key risk to monitor: will procurement and construction costs increase materially since project first announced in end-22 when the design is complete and contracts let in FY25? A 25-50% cost blowout would not be unprecedented. While that would not be good, it is not really that material, overall.)
4. Revenue & Markets
RoW sales are becoming material "from 16% of total sales to 24%"
Now have sold product into 37 countries
Key market details (not all presented but covered in voice-over):

After not having raised prices in US for several years, there is now an agreed approach for price revisions
In the US, “narrowing the gap” to the market leader in the “difficult burns” category
BARDA trial now 91 patients enrolled; 1st patient enrolled in India (2 centres approved). Looking at options with FDA and BARDA. Base option is to get to 120. Enrolment expected to be complete by May. There will be announcement when it is decided how to close out the trial to a meaningful close. Also working with another FDA agency to see how “real world” data can be sed to give added claims into the trial.
Strong growth in ANZ where already #1 in burns is largely outside of burns
India – “half a dozen tenders” under way. “Getting good soundings.” “Very optimistic that in the very near term we’ll have something to say.”
HK also continues to “trade well”. China – pathway identified, but not yet going beyond HK. Developing plans for extending into Shenzen area (GBA).
Germany: market leader in “Advanced Dermal Substitutes” (4th largest market after US/UK/ANZ – note: Ger. is a distributor market)
Turkey – large initial sales
Middle East – sales driven by a physician who relocated to ME from East Coast US and wanted the product
Japan – have a partner defined and a lead KOL. Working to see if data already submitted to US FDA can be used for submission in Japan with MoH, KOLs and reimbursement agencies.
New demand arising from war zones in Ukraine ($1.2m sale in February - not 1H; “we believe another coming”) and Israel. They believe they are getting some sales orders from other countries that are ending up in these locations, as well as charities.
Several ongoing discussions with charities, UN agencies, Gates Foundation, WHO, MSF, etc. to help get the product to countries that otherwise can’t afford it.
5. Organisation
Headcount +64 on PCP, but only +19 in 1H FY24
Plan for 2H FY24 from 237 to 260 +(23-25)
- US sales: currently 75 (65 reps + 10 managers) going up to 85
- ANZ sales
- R&D and Manufacturing
- Back Office: now have the full teams and scalability
Completing the build-out of the senior management team
- HR lead has started in last few weeks
- Chief Medical Officer joining – leading on FDA and BARDA conversations
- General Counsel about to be appointed
- Close to appointing APAC lead, for entry to China and Japan, and other countries
6. Clinical Developments
My key takeaway: It is clear here is the potential for many years of development leading to new platforms and multiple groups of new indications. Most of this is clinican led.
A key observation is just how important the customer-led (clinicians) work is. (My Note: Just reflect on the following points and consider the tiny R&D budget. I don’t think I’ve ever seen this before in healthcare. There is so much upside to come.)
DFU study: stopped after 25 patients because not getting right wound debridement. Protocol to be re-written and brought back in-hospital (rather than outpatients) to get great consistency needed for a successful trial. Expecting to focus more on to limb salvage – new trial, Announcement on trials coming in a few weeks
Prof Marcus Wagstaff trained 30 surgeons in UK who then took the knowledge onwards to Ukraine
Much clinician-led development taking place into new indications; 230+ publications (214 at FY23), “literally across the entire clinical spectrum”
Customers proposing BTM could be a replacement for allografts (papers on this). Potential to upshift and replace grafts
Several authors proposing that BTM could be a good solution in low and middle income countries where other technologies are constrained
Overall global markets outside US and W. Europe and a few market in APAC “on the fringes” (hey! Swami, that’s no way to talk about ANZ) – we are focused on sustainable, global growth
MTX rolling out in US – demonstrating great outcomes in open abdominal and dehisced wounds. Will start compiling evidence to allow MTX to be rolled out globally. Focus has been using MTX with “expert clinicians” on complex applications, and expecting wider roll-out from July.
Working to be differentiated in connecting KOLs across the world who can teach other surgeons in how to treat acute complex wounds.
$PNV already recognised in burns and trauma, and now looking to go beyond these into:
- Oncological resections of head, neck, scalp, skin and oral cancer
- Potential to use in infections (necrotizing fasciitis and hidradentis) where competitor products cannot deliver
- Getting into complex vascular space to save limbs from being amputated
Developing an implantable platform in hernia and breast. Still not happy to share timelines, but happy with the feedback getting from clinicians. Addressing how to build a “sustainable platform in the implantable space”. (Sounds like still some time off, but I still think this is OK given the growth potential of BTM and MTX)
Working on developing Novosorb Mesh product, currently bench testing, testing with animals, and sharing it with clinicians to establish their expectations on added strength and flexibility. Work is being done to compare with the market leader.
Disc: Held in RL and SM
Three updates this morning - seems about right.
Superbull Macquarie slowly coming into line!
While I am not running my model update until FY, I'll tweak my val. up by 5% just so as not to be too out of whack with the new information.

Disc: Held in RL and SM
Dermal repair company $PNV reported its 1H FY24 results this morning. The major elements of 1H have been pre-released in the “Trading Result” announced over a month ago. This set out the sales results, which were very strong, as well as anchored the key financials. So, I expect that today will be less about the result itself and more about the trajectory towards the FY. More on all that below.
Their Highlights
The half year audited results attached to this release show:
- Record 1H FY24 sales of A$42.2m up 54.9% on STLY of A$27.3m.
- Total revenue including BARDA of A$48.8m up 65.6% on STLY of A$29.5m.
- Strong growth in U.S. achieving record sales of A$32.2m up 41.7% on STLY of A$22.8m.
- ROW sales of A$10.0m up by 122.2% on STLY of A$4.5m including strong performances in ANZ, UKI, and the Middle East, also growing sales in India, Hong Kong, and Canada.
- The Group recorded a net profit after tax of A$2.7m (1H23: A$3.8m loss).
During the Period, the Company’s other key initiatives and achievements include:
- Exceeded $8 million monthly NovoSorb BTM sales for the first time in November ($8,810,000).
- Appointed Chief Medical Officer and Chief People Officer.
- Additional funding of US$10 million from the Biomedical Advanced Research and Development Authority (‘BARDA’) for the pivotal trial of NovoSorb BTM in full thickness (third degree) burns.
- Passed the mid‑way point for the recruitment of the pivotal trial, with 90 patients currently enrolled.
- Increased sales teams and customer base globally, 861 hospital accounts and 237 staff.
- Progressed the product pipeline for NovoSorb BTM and NovoSorb MTX and developed surgical mesh prototypes for hernia repair.
- Finalised concept design and commenced detailed design of additional, new manufacturing facility in Port Melbourne.
Context for today’s result (you can skip this as it is a little self-indulgent!)
SP action for $PNV is a rollercoaster that sometimes defies belief, bearing little relationship to the business fundamentals. A bit like riding a rollercoaster in the dark, where you cannot see if the next move is a soaring climb, or a plummeting fall that risks bringing the contents from the last fast food stall you visited before the ride back up! Entertainment value is added by Ride Master David Williams – red-jacketed, red-faced, and gesticulating, wildly to “Roll-up. Roll-up” as he waves at you with a 30cm piece of BTM, while CEO Swami in the background cooly explains the genius of the engineering that allows the ride to function, assuring punters of their safety.
$PNV reached its most recent low point in October. This was driven by a mixed market reaction to the growth of the cost base supporting the global acceleration (clearly signalled at the 2022 capital raise), raising doubts as to the path to profitability, not helped by Ride Master DW saying he didn’t care about profits.
However, since that time, four factors have driven a sustained 5-month uptrend of +80%:
1) the release the $8m/($9m record month in December,
2) the January pre-released Trading Result with preliminary bottom line,
3) the record single sales order of $1.2m and
4) broader, macro-risk-on.
But this is a rollercoaster, and we are still some way off the lofty heights of $2.69 reached in the run-up to 1H FY23, so you never know how far the climb continues or whether we temporarily lurch downwards once more before recovering.
Through all this, I try not to let the “Buy, Buy”, “Sell, Sell” trader-analyst-fundies distract me. They’re not much help really with an average Target Price of $1.95 representing a wild range of $1.00 to $2.90 – materially down on 12 months ago ($2.53, $1.90 - $2.90).
My model is at $2.00 ($1.63-$3.30) with my eye clearly focused on the disproportionate upside potential, even though I have also come off my position in Sept-22 ($2.46, $1.62-$3.28).
So, with the scene set, what do I make of today’s result?
My Analysis
On the release David has said: “There is little new here that was not in our 22 January announcement. It was a great half, but we have moved on. There is a lot to talk about that has happened since 31 December, which we expect to talk about during our investor webcast on 27 February.”
In other words “Roll-up Roll-up to the David, Swami and Jan show at 1pm AEST today!
Important Note: My analysis below may differ to what is presented today. In fact, it will, That is because $PNV typically make various adjustments and report underlying numbers in their presentation, whereas I stick to the audited accounts. That said, no-one audits my analysis. So, all care, no responsibility!!
Revenue
There is nothing to add on sales to the detail I gave in my straw on 22-January. Revenue (which includes BARDA) is up 65.6% - a slight acceleration from the PCP. Sales are up 54.9%, with the US up 41.7% and RoW up 122.2%, with sales in several new markets.
With a FY revenue consensus of $101.4m, revenue in H2 needs to hit $52.6m, which would be growth over the pcp of 42.0%. So, how likely is this? Well, H2 growth rates in FY22 and FY23 have been 43% and 56%, respectively. And looking at the last three years, there is no clear 1H / 2H trend. H2 FY24 also has the boost of at least 1 large order to Ukraine valued at A$1.2m. And with the recent impetus in RoW from the expansion of the global sales and marketing footprint, everything points to a strong finish to the year. My model is for FY sales of $105m for the FY. But I am a $PNV bull, so DYOR!
Gross Margin
Gross Margin comes in at 95.9%, compared with 94.5% in pcp. Overall,it is in the usual ballpark of 90-96%. This is expected as the direct fixed costs of the current facilities are recovered over progressively increasing volumes. However, when the new facilities come onstream in FY25, I expect %GM will drop back sharply, as the new facilities have been sized to support sales up to $500m p.a!
Still, compared to the competition in dermal repair, $PNV has an extremely high %GM.
Opex
Opex (excl. D&A) grew 46% from $30.6m to $44.6m, a slower rate than 107% in the PCP. Importantly, it is now growing at a slower rate than revenue. Opex is now 91% of revenue, down from 104% in pcp. Yay!
This moderation was expected for two reasons, First, a major expansion of the workforce occurred during FY23, following the capital raise, to pursue the broader global sales strategy. While expansion has continued in 1H24, there has been a greater focus on execution. The second reason is that the FY23 comparison was distorted due to some items relating to former CEO compensation. In the presentation this is one of the “underlying” corrections that Jan has made.
That said, corporate costs have expanded significantly, given the senior hires indicated above. However, $PNV is not a truly global business, and you need functional heads capable of delviering their roles in that context.
Within Opex, R&D continues to expand. This is important and welcome, as without ongoing innovation $PNV can never become a long-term winner in dermal care. Management seem to be applying capital discipline here, holding R&D/Revenue at 10%, in order to deliver their commitment to getting to profitability.
So, overall, I am very happy with the progression of the Opex profile.
Profit and Cash
By my calculations, EBIT is $1.1m (up from -$3.8m) – an improvement of $4.9m.
NPAT is $2.7m, up from a PCP loss of -$3.8m – an improvement of $6.5m, assisted by the Tax refund of $1.6m.
So, well done David, Swami and Jan and team. You are on track to delivering your commitment at the FY22 Capital raise to be profitable in FY24.
Cashflows align quite well with the financials. We are now just Operating Cashflow positive, at $0.58m compared with -$2.73m in PCP. And by my measure of FCF (which includes all capex), they are close to breakeven at -$0.48m.
It is worth noting that capex has increased from almost nothing to $1.1m, as the build the new production facilities. This should be expected to ramp up significantly over the next 12 months. So, hopefully, management will guide on that in the presentation this afternoon.
The balance sheet is strong with Cash and Equivalents at $45.58m, and debt at $2.2m (current and long term) is negligible and being paid down.
My Key Takeaways
Solidy on track. Continuing strong revenue growth and management demonstrating cost discipline to meet their commitments. May it continue.
Valuation
I’ll update my valuation after the FY24 results. For now, I am content to stay at $2.00 ($1.63-$3.30)
However, I can say that if execution continues in this manner, I'll be upgrading as the downside cases in my model start to fall away.
Disc: Held in RL and SM (with high conviction)
DW as ever quick to make sure everyone on the mailing list gets the Macquarie update on $PNV following yesterday's trading update.
Macquarie are the most bullish of the bulls, and lifted their $2.70 Price Target to $2.90 on the trading update that came in 8% ahead of their revenue number.
Accordingly, their FY revenue forecast is $103.0m, which is about where I am.
The Macquarie price target is well ahead of my central view (update this morning to $2.12) as they are more aggressive on revenue growth and profitability in the early years. I can easily get to the Macquarie valuation, as my upper scenarios are north of $3.00, so they are in my view well within the realm of reason.
$PNV remains one of my favourite holdings. After a few years of trading sidesways - and down more often than not - 2024 could be a breakout year for the company as it moves to deliver its first NPAT - particularly if it can maintain >50% revenue growth, which appears likely as super strong ROW progress offers the prospect of offsetting any moderation of US growth that may occur over coming years.
Disc. Held in RL and SM
$PNV has issued its 1H trading update, which it usually does around this time.
Their Highlights
• Total global revenue including BARDA was A$48.8m, up 65.6% on STLY of A$29.5m.
• Record 1H24 sales of A$42.2m up 54.9% on STLY of A$27.3m.
• U.S. 1H24 sales of A$32.2m up 41.7% on STLY of A$22.8m.
• Rest of World sales of $A10.0m up 122.2% on STLY of A$4.5m including strong performances in ANZ, UKI, and the Middle East, also growing sales in India, Hong Kong, and Canada.
• BARDA revenue of $A4.9m up 133.1% on STLY of $A2.1m. Currently 83 patients enrolled in pivotal clinical trial.
• EBITDA, EBIT and NPAT all positive. EBITDA $1.9m, up $4.4m on STLY EBITDA ($2.5m)
• Underlying EBITDA $3.6m (excl. non-cash items)
My Analysis
With total revenue up 65.6% to pcp, we are now seeing the expected acceleration. (The last two pcp comparison for 6m reports have been +56.3% (2H23) and +62.1% (1H23)), which means we are seeing the benefits come through of 1) the early 2023 expansion of the US salesforce and 2) the entry intro new territories.)
Digging into the numbers, this growth was only possible due to the expanding BARDA trial, without which we'd have seen growth of 55% (still decent), driven by US with 42% (Recall US FY23 growth in cc was 34.0% in CC, so it will be interesting to see the cc number when the full report comes out. However, 42% should be good as it comes at a time of greater fx stability and potentially even a stronger $A in Q4).
ROW ticking along nicely too at 122% off a small but increasingly material base.
It is also good to see the report for "EBITDA, EBIT and NPAT all positive". The NPAT bit is the key commitment made at the last capital raising, which has been re-affirmed at each report, even though DW claims not to be concerned about.
Overall, provided $PNV maintain a reasonable cost discipline then the second half could lock in a decent maiden profit.
$PNV appears on track to my forecast of $102m for the FY.
Overall, on track, and in line with expectations.
Disc: Held in RL and SM
Sure enough,.... another "record month".
First $A8M sales month and $A9M revenue month
PolyNovo announces record monthly sales of $A8.8m (unaudited) for November 2023.
Highlights:
The U.S. business grew strongly, with monthly sales of $A6.1m (unaudited), up circa 74% on STLY
Rest of World had monthly sales of $A2.7m (unaudited), up circa 290% on STLY. There was strong growth in UK/I, ANZ, and the Middle East.
Total group revenue for the month, including BARDA was $A9.5m (unaudited), up circa 110% on STLY
My Analysis
With previous results announced for July and August, this has $PNV on track for the year, as far as your can tell with this type of cherry-picking reporting methodology. Strong numbers across the Board.
Disc: Held in RL and SM
Nothing new in here, but nonetheless a succinct summary of progress over the year.
DW's address to the Bell Potter Conference.
Disc: Held in RL and SM
Just online at the $PNV AGM as I love the DW Circus, coming today from the MinterEllison Big Top in Melbourne.
Interesting question to DW first up from a shareholder on why $PNV doesn't provide regular quarterly reports. I've scanned ahead the MD and CEO's report and, lo and behold, no reference to the last quarter's revenue, highlighting only the August $7.7m.
David has predictably defended the practice, but he can't escape that the market will likely interpret this means that Q1 was not such a strong result (otherwise the'd report it). The cherry-picking will continue and with it, in my view, the SP volatility!
Not reporting is reporting, when you're a cherry-picker!
Why I Wish He Wouldn't
I couldn't help myself write this straw on why I wish David Williams would change his investor relations behaviour. The commentators I have seen since yesterday's trading update announcement have made three statements about yesterday's release:
- Why report two months sales data compared to pcp, when the quarter end is a few days away? (There wasn't even an $8m month to at least be consistent with previous patterns of reporting) Particularly when there isn't really anthing to disclose.
- The growth results are strong, fair enough. (Particularly given the languishing SP)
- It doesn't change views on valuation
That's pretty much where I was yesterday in my straw.
But, you say, the market has moved up 14% in the two days. True, but the stock is still down 50% from its current 12m high in February.
So here is why I don't like David's "cherry picking reporting" and it needs a graph to explain. Note that the numbers below are made up, as they seek to illustrate a general point that is applicable to $PNV. The calculated rates of growth are relevant, however.

So here's what it shows. (Don't worry about the absolute numbers, I just picked 100 as an arbitrary starting point!)
Blue Line (acutally a curve), starting at June-22 at 100, this line grows each month at a compound monthly rate of 3.99% or 60% p.a. - which is in the ballpark of $PNVs current annual sales growth.
Orange line: Jul-22 and Aug-22 are depressed below the trend by 20% - indicative of the lumpiness we know exists month to month in $PNV sales. Let's assume we had two bad months at the beginning and two strong months at the end. The "lost" sales from the beginnining are added back in above trend in Jul-23 and Aug-23. So the total sales over the 14 month period is the same for the Blue curve and the Orange curve. It's just that the blue curve is perfectly smooth and on trend while the orange curve is lumpy at each end.
This is my suspicion of what could be going on. Two soft months cherry-picked at the start and cycling two strong months at the end for pcp comparison.
Red Line: So, let's do the maths. What's the ANNUAL SALES GROWTH rate from Aug-22 to Aug-23 (or Jul-22 to Jul-23)?
Blue Line = 60% growth. Red line (for orange curve) = +125%. Over double the underlying growth rate.
That's how misleading annual growth numbers can be if you cherry pick your data-set.
Which is why the high reported numbers in yesterday's ASX release from a management team with a reputation for cherry-picking data shouldn't necessarily result in any change in our view of the underlying value of the business.
Of course, the good news is that, even if the release represents "peak cherry picking", its does mean that underlying revenue growth is still in the region of 50-70%, whereas the market is expecting +42% this year and +34% in FY25.
As a result, no analyst upgrades should be expected, apart from the bears who have a SP <$1.50 or so. (There is one in my dataset with a price target of $1.08. Maybe they'll wake up one day).
Life would be much easier if we got any of the following:
a) Monthly sales, every month or
b) Quarterly sales, every quarter or
c) HY and FY results. Period.
Of course, I could be wrong, and perhaps the underlying trend is stronger. I hope it is. But that's the problem with management who are too promotional and inconsistent in how they report. You just can't tell.
If I wasn't so high conviction on the product and the business, I think I would have given up long ago due to exhaustion.
No doubt, the David Williams Circus will continue to roll on. So,... roll up, roll up.
Disc: Held
So here is the annoucement:
Key messages
- August Revenue of $7.7m up 119% in August pcp and 93% YTD
- YTD revenue is $14.9m vs. $7.7m in prior year
- US sales in first two months of FY24 were $10.6m up 85.3% over pcp
- RoW is $2.4m up 78% over pcp
I can't see any promised "upgrade" anywhere.
More detail is given in the release.
My Analysis
OK, so FY24 starts with 2 strong months, in what is normally a lumpy sales business month to month. Hopefully, as it scales, lumpiness should continue to smooth out and maybe we can get to some regular 6-monthly reporting.
These guys are relentless in cherry-picking good news periods and issuing a release, which to me says nothing other than they are peeved that the SP is as low as it is and they are trying to correct that. I think that is a fruitless, pointless effort. The half-year and full-year results will do the necessary work over time. OK rant over, as ranting is also pointless.
There is of course good new in here. In my recent assessment of their annual results, I commented that the US appeared to be slowing with only 34% constant currency sales growth in FY23 over FY22, in a period when the sales force was significantly ramped up. Even though we are not seeing a constant currency comparison in today's release, a two-month burst of 85% growth over the pcp is very good news and would be very strong even after correcting for $A to $US.
In most of my valuation scenarios, my expectation was that the US FY23 over FY22 result was a softer result than the underlying trend, given lumpiness of individual periods. Today's announcement lends some support to that case.
Key Takeaway
I've recently published a detailed, updated valuation of $PNV. Today's announcement contains no material new information, but it supports my overall bullish thesis. If anything, it nudges the likelihood of my lower valuation scenarios of <$2.00 lower in likelihood and is in-line my more bullish view of the business. No updates required as a result of this. I remain a high conviction hold.
No doubt the market will take the short-term sugar hit. But one day the SP will move on from these low levels and never return.
Disc: Held in RL (3.8%) and SM (14.6%)
David has kindly emailed around the recording of $PNV's presentation at the Bell Potter Emerging Leaders Conference 2023. Here it is for those not on the mailing list.
https://youtu.be/scQoF-zaZrY?feature=shared
Many are stories we've already heard (some again and again ... and again).
But there are a few nuggets of interest
India
DW says they are now generating sales in India. In a separate statement he says they want to hold consistent pricing internationally. "Even if we made the decision to go into India at half the price, that we're charging in the US or Australia, we'd still be making a margin in excess of 80%."
In my recent valuation, I have been more bearish on emerging market pricing, running scenarios at 25%, 30% and 35% at modest volumes.
I couldn't imagine that DW would still be using a 50% benchmark (implying its a lower bound) unless they were generating sales in that ball park. Its difficult to avoid reading the tea leaves, and I probably just have to be patient until Feb/Mar 24.
Germany & Europe
In providing some further details about Germany, David confirms Novosorb is the standard of care there. This was also shown on a recent presentation. He notes that although their preference is to market directly, they are using a distributor in Germany because the distributor already has a complementary product. Perhaps this goes some way to explaining FY23's +189% German sales result.
DW noted that they are now also selling in France and Spain.
USA
He said that the product is getting more traction with diabetic foot ulcer and venous leg ulcers in the USA. He said they are considering hiring a specialist sales force for the podiatric surgeons OR are considering a specialist distribution deal. Again, Swami spoke about this pretty much on day 1, and a year later they are still mulling this one over.
Focus & Blue Sky
The other key message confirms that they are focusing on broadening the clinical applications of the current products, or rather, staying close to the surgeons who are leading the innovation.
David finishes by flashing the "blue sky" applications. We spent a lot more money on R&D in the last two years,... we used to have a man and his dog ... now we must have about 8). "We've got people developing new products for us",...with an update on the second patient now treated with the diabete drug elution product at Royal Adelaide Hospital.
Of course, the tangible measure is when DW announces the next milestone month, which would be $9m.
In summary, there is nothing really new here, but I like listening to understand how the messages and emphasis are evolving.
Disc: Held in RL and SM
I have finally rebuilt my valuation of $PNV. The reason for the challenge in doing it, is that the change in sales & marketing strategy since Swami came onboard as CEO. This has driven the need to create an entirely different way at looking at both sales growth and contribution margin - the dual push on developed and developing markets means that modelling performance by averaging key metrics means you lose sight of what is going on. So the model needed a total rebuild.
Estimated Value $2.00 Range [$1.63 - $3.30]
Based on 9 scenarios, I arrive at an expected value/share of $2.00, and an estimated p10%-p90% range around this of [$1.62 – $3.30].
This is a significant downgrade from my prior valuation $2.46 [$1.63 - $3.28] from September 2022. However, what is interesting is that the upside and downside ranges are largely unchanged (albeit also reduced about 10% given the passing of a year.)
Revenue Growth
Revenue growth is modelled driven by sales force expansions aggregated into separate Developed Markets and Developing Markets buckets.
- Developed market performance starts with average sales per rep of $0.811m p.a.
- In Developing markets, realised prices are modelled at 25%, 30% and 35% of developed market prices (see “Commentary” at end of this Straw). At this stage we don’t really know what the net sales values will be in these markets.
- Costs and Revenues are subject to constant annual inflation (2.5% developed markets and 5.0% in developing markets.)
- Like-for-Like growth: As more and more indications for Novosorb become accepted and applied, the value of each account is expected to increase, and each saleperson become more productive by accessing more clinician-customers at each hospital visit. Research publications indicate that we are still very early in this process of adoption. On average, an annual “Learning” growth factor is applied to each Sales Rep across a range from a minimum of 3% to as high as 7% across different scenarios. This is one of the most important value drivers. (Hence DW is actually getting investor to focus on the right information in emailing around clinical review studies showing the increasing breadth of clinical applications.) In truth, I have no idea what the appropriate learning rate is. However, as an indication at the expected valuation, a 1% increase in annual learning at the mid-range leads to a $0.30/share increase in valuation. Wow! The best way of tracking this metric is by continuing to focus on the number of sales reps in the USA and the value of US sales. (Swami has said from day 1 that $PNV has to stay close to the physican customers to understand how they are innovating in their use of the product. That's why.)
- Sales and Marketing costs have been estimated by calculating: benchmarks market rates, adding allowances for super, health insurance, seniority mix, incentives and applying a a ratio of one marketing FTE per 7 sales FTEs. A non-FTE sales and marketing expenses factor of 12% revenue has been applied (this covers everything from advertisements, free sales, to conferences and flying opinion leaders around the world.) This is likely to be on the high side, but I have modelled a range of expense growth scenarios to accommodate this uncertainty.
- In developing markets, a 2023 cost for a sales rep. has been estimated at $40k p.a.. This is probably on the high side for current costs in India (as quoted by DW), but over the 10 years the model assumes expansion to all major cities in middle income countries across Asia, Central and South America and the Middle East. The valuation is not hugely sensitive to this assumption.
The output from the Revenue Growth and Sales and Marketing modules is shown in Figure 1 for one scenario, below.
Figure 1: Revenue and Sales and Marketing Headcount for one Scenario

It is worth commenting that I have followed a cautious approach to the build-out of developing markets that may not stand the test of time. For example, in 2023 a team of 22 people has been established in India within months of market entry, with as yet no material sales. By contrast, the combined UKI and ANZ sales teams number only around 13, with combined annual sales of around $7-8m.
As a result, I follow a more cautious build-up in the developing market sales force over time. These reach a total workforce of between 160 to 320 by 2033, with a contribution of 13% of total 2033 revenues in the most conservative to an upper case of 26% in the most aggressive case, with 20% shown in Figure 1 above. This is somewhat at odds with the rhetoric we’ve been hearing from Swami and David over the last 12 months. However, I want to see proof that developing markets can make a meaningful margin contribution before setting too much of my investment thesis against that.
It is getting to grips with the new prominence of India (in particular) and developing markets in general that has given me the most pause for thought in modelling the updated strategy.
In my earlier September 2022 valuation, I assumed a more aggressive approach to Europe and other high value markets like Japan, South Korea, and Taiwan. So, as far as my valuation is concerned, the change of strategy has lopped $0.40-0.50 off my valuation. That is the price of me NOT sharing Swami and David’s conviction. I may well be wrong and will be happy to be so proven!
Cost Growth – R&D and G&A
In all scenarios I assume a moderation in the % rate of cost growth of the company, which over recent years has really been in a start-up mode. David and Swami should demonstrate this disciplined as they are now under pressure to breakeven this year and show a meaningful profit in FY25.
While expenditure on R&D and G&A both grow strongly throughout the 10 years modelled, I assume $PNV remains a highly focused company selling only variants of the Novosorb product (BTM, MTX, etc.). This allows for a highly-focused organisation and cost structure which achieves exceptional net margins.
Figure 2 shows the projected evolution of expenses as a % of Revenue. In Scenario 6 (shown), the 2033 Net Margin is 32%. The range of modelled scenarios yield net margins are 30-36%. This compares with a Net Margin in 2022 for competitor $IART of 12%. Good net margins in medical devices are more typically in the range of 10-20%. So how can this possibly be?
The answer lies in the high gross margin (93% in FY2023 for %PNV). Medical device companies more typically achieve gross margins in the range of 50% to 65%, with $IART achieving 64% in FY22. Thus, $PNV has a very material Gross Margin advantage, providing headroom over the competition of a full 25-30%! That's massive.
This positions it well to respond to competition, as other synthetics will inevitably emerge over time. (To be fair, David has been banging on about this for the 5 years+ I've been following the company, and it was only over the last weeks when I did some detailed industry benchmarking, that I realised how important this is.)
Figure 2: Evolution of Expenses (Scenario 6: $2.16/share)

Looking at the % expense/revenue structure modelled in the above scenario in 2033 and comparing it with $IART in FY22:
- R&D of 8% at $PNV compares with 6% at $IART
- SG&A of 36% at $PNV compares with 40% at $IART (with the latter a much more complex business)
Of course, $IART is a much larger company with a much more diverse product portfolio, so the comparison is limited. However, what $PNV lacks for in economies of scale, it can be argued to recover in its narrow product focus.
(*As a "post-production note": I've probably been a bit aggressive on $PNVs G&A, so will address this next time. In the case illustrated above, if 2033 G&A/Revenue is more like 12%, then value is reduced to $2.04.share)
Total R&D spend over the 10-year modelled period amounts to over $200m (nominal), however, no new products are assumed beyond new variants of Novosorb, such as MTX. It is assumed that significant funds should be spent in supporting studies to broaden the range of product indications, and providing tailor variants, like MTX.
New products for applications in breast, hernia, other internal procedures, and drug elution are all still assumed to be “blue sky” upsides, even though this is where the lion's share of the R&D spend will do. So, there are significant potential upsides for the business that have not been contemplated in these valuations.
This level of R&D investment without an explicit revenue stream justifies the continuing value annual cashflow growth beyond 2033 of 5% p.a. (However, this might be conservative if $PNV is successful in positioning itself in developing markets. Here, although revenues are low by comparison to developed markets, growth rates are very high.)
Gross Margin (GM) Evolution
Historically, $PNV has achieved %GM ranging from 91% to 95%. With the proliferation of product variants and lower price realisations in emerging markets, I assume 93% represents a cap on future gross margins. As the new facilities come onstream in 2025, lifting sales capacity to $500m, I assume %GM falls to 91% as the existing sales bear the fixed costs of the larger production facilities, before returning to higher values as plant utilisation ramps up.
Capex
Capex has two drivers: "base PPE" capex and investment in new production facilities.
Base PPE capex is assumed to grow proportionately with sales.
Expansion of new production facilities occurs in two tranches: $30m capex to scale up to $500m total sales (current reported cost is $25m), and I've assumed a further $40m to scale up to $1000m total sales.
Timing of expansion capex is driven by revenue growth assumptions.
Common Assumptions
WACC = 11%
Inflation = 2.5% (5% in developing markets)
Effective Tax Rate = 30%
Shares On Issue = 1% growth p.a.
Capital Leases grow proportionately with G&A
Working Capital grows proportionately with Revenues
All R&D expensed
Cash Flow Growth in Continuing Value Period = 5% p.a.
Valuation has been performed on a 100% Equity basis - no debt. As the business scales it will make sense to put in some long term debt. $IART by comparison has Net debt/EBITDA of about 2.4x. However, at this stage in its evolution, I prefer to consider the business as 100% equity funded.
Commentary on the Analysis
Developing Markets
With the current push on India and with China to follow the Hong Kong beachhead (3 out of the 4 major hospitals already signed up), $PNV is entering new territory, so all the developing markets analysis is a placeholder. I have, however, tried to err on the side of conservatism. At this stage we have no idea what contribution margins might be achieved.
So let me put this my reservations in context, because I suspect Swami and David would disagree with my analysis. The US medical devices market is c. $164bn p.a., around 3.4% of the US Healthcare Spend. The Indian medical devices market is around $6bn p.a. That’s less than 4% of the US market. In the US, average annual healthcare spend per person is c. $15,000. In India, it is $101.
I understand the clinical and humanitarian imperative to bring lifesaving and life-enhancing treatments to developing markets. And I support it. My point in writing here, is that I can’t at this stage model the economics for $PNV in developing markets with any confidence. The strategic shift in the priority of market rollout has chopped about $0.50 off my expected valuation. Only time will tell it that’s reasonable.
US
The US is a potential concern. With a salesforce now in the region of 80 in the US (up c. 50% on end FY22) to have seen only 34% constant currency sales growth in FY23 over FY22 raises a question mark. None of the analysts appear to have picked up on this, so far as I can tell. But it is the key result I'll be focused on in 1H FY24.
It is this question-mark which leads me to be cautious on the ultimate potential of the US market. (I max-out the US sales force at 150 reps. at about 2030. If US sales growth of >30% p.a. can be sustained for a few more years, then I will revise this upwards next year.) This upside possibility is not fully accounted for in my range of scenarios.
Europe
For me, Europe remains the untold story. UKI was a big surprise in FY23. Growth of +169% means this must be getting to total of around $3.50m-$4,0m in sales. (Of course, by comparison to ANZ, an equivalent penetration would be more like $12m at a comparable stage, so there is a lot further to go.) Germany grew +192%, but this is via a distributor, and off a very low base as to probably not be meaningful.
Still, UKI and the EU is a high value market of some 450 million people and, even though a smaller $ market in aggregate than the US for medical devices overall, it is still very significant and largely untapped by $PNV. In all my scenarios, I assume a direct sales model for Europe but a slower build-up over the 10-year period. So, there is the potential for upside to all scenarios, particularly if the latest UK experience leads to management getting more serious about Europe. (C’mon on Swami, you can do it!)
Ongoing Visibility
It looks like $PNV are going to disclose sales on a US and RoW basis only going forward, with scatterings of informal disclosures to highlight individual market milestones or successes. I don’t like that because RoW is going to be a blend of 1) long term developed markets (like ANZ and UKI), 2) new developed markets like (EU, Canada, and Japan) and 3) developing markets, primarily India and China. This is going to make it hard to track how things are going for us analysts. However, the good news is that the US will give good insights into clinical adoption, market penetration and competitive positioning … and for the rest, we’ll just have to extrapolate! Hopefully, we will soon be able to get intelligence on realised prices and volumes in India.
Range of Modelling Outcomes
Below in Figure 3 is a summary of the valuation outcomes. I’ve equal-weighted each of the 9 scenarios, and from the graph been able to read off p10, p50 and p90 estimates of value.
Table 1 summarises some of the key valuation metrics and multiples.
What I do know from all the modelling is that higher valuations can easily be achieved, and it is much harder to drive downsides much below the $1.40 - $1.50 range. This is the same conclusion I reached in 2022.
So, I am comfortable that today $PNV is significantly under-valued. It remains my largest SM position and at the top of my conviction list in my high risk holdings.
In RL, the great progress of $WTC, $ALU and $TNE (plus my doubling-down on $RMD against the shortselling-thesis), means it now only sits in 6th position at 4%. It is tempting to add more at these prices; however, it remains a risky proposition with a lot of execution risk. Often the best investment strategy is to do nothing.
Figure 3: Outcome of Valuation Modelling Scenarios

Table 1: Summary of Key Valuation Metrics*

* Note that the High value of $3.62 is higher than my quoted upper limit of $3.30. This is because the valuation range is read off the above chart at the p10% and p90% levels, and ignores any extreme individual valuation results.
Disclaimer
The above analysis is not valuation or investment advice and is for my personal purposes only. It is provided for information and to stimulate debate. The analysis is not validated to be free from errors.
Disc: Held in RL and SM
I know a few other StrawPeople follow $PNV closely. So this is a question of detail for them.
I've been trying to get my head around the statement: "U.S. NovoSorb sales $46.1m up 44.6% (34.0% in local USD)"
We don't get consistent reporting of numbers, so I have constructured the following table for US Sales in A$ and US$. (Warning - shaded numbers are my calculations and have not been reported by $PNV and may be wrong, particularly given that I am using an annual average FX when volumes vary significantly through the year).

The numbers I am focused on is the constant currency USD sales, %y-o-y difference.
My estimates of the FY-end US Sales Force (FTE) are as follows (note: not reported)
2021 = 36 (reported)
2022 = "54" (At end 2021, DW said Ed would add another 20,... I assume he came up a couple short)
2023 = "75" (And then the same again in FY23)
Note - the last two numbers may be inaccurate, but as far as I can tell, they haven't disclosed US sales force numbers for a bit. DW has thrown some numbers around, and there is as far as I can tell now a sizeable non-Sales US headcount. However, directionally, the numbers make sense given 218 total reported at 30-June-23.
Forget, the fine detail, but doesn't this point to a significantly slowing of US$ sales/ salesperson? We know it takes a year or two for new starters to ramp up their sales volumes. So, given the big increase in numbers over the years, shouldn't there be a sizeable lag effect as productivity of sales staff added 2 years ago continues to grow. So, however you cut it, incremental USD sales of $7-8m FY22 to FY23 appears light.
Maybe something structural is happening. Maybe the initial workforce covered high-use burn clinics, and now incremental workforce are hitting lower returns, with new accounts being less valuable in general hospitals. (By the way, that is an entirely rationale sales and marketing strategy.)
IF (and it is a big if) this is real, its going to masked in FY24 by big numbers coming form emerging markets, and we won't find out until some time down the track that saleforce productivity or market penetration is flattening off.
I feel like I am having to play Hercule Poirot here, and just wish they were more consistent in their reporting. But before I fire off a missive to DW, has anyone else sensed anything?
Has anyone else had a look at this?
$PNV reported their FY23 results, and before heading into the David and Swami show, I am reporting my quick take.
Top line: strong revenue growth slightly ahead of market expectations at +59% (alebit behind my model), but with a significant expansion in costs, with total expenses up +66% largely driven by expanding the sales force into new markets. This drove an increased net loss of A$4.93m (FY22 A$1.19m), which they go on to say is essentially flat after adjustments (departed executives ... the gift that keeps on giving).
Their Highlights
- Total revenue including BARDA of A$66.5m up 58.8% on prior year of A$41.9m
- Strong growth in U.S. achieving sales of A$46.1m up 44.6% on prior year of A$31.9m
- ROW sales of A$13.5m up by 133.9% on prior year A$5.8m
- The Group recorded a net loss after tax of A$4.93m (2022: $1.19m loss).
- The loss in the prior year FY22 included the reversal of A$4.7m in share-based payments from the forfeiture by the previous CEO and COO on their resignations. The underlying net loss after tax excluding non-cash items is A$2.32m (2022: $2.00m loss). At year end the business had A$46.8m cash on hand.
- Excellent sales growth can be explained by the genius technology that is NovoSorb BTM and NovoSorb MTX, and by an increased sales force and geographical expansion. However unprompted surgeon engagement around the world in trialing the product, and publishing and presenting their results and developing new uses for the product has been amazing.
The Company’s other key initiatives and achievements include:
- First $7 million sales month in May 2023 (May 2022: $3.3 million), Total Revenue $8.3m in May 2023
- $53m capital raising
- 510(k) clearance from the FDA for NovoSorb MTX and saw first sales to plastics and reconstruction
- Entered Hong Kong, India, and Canada markets and saw early sales
- Grew the U.S. team from 54 to 93 and increased U.S. customer accounts from 189 to 299 hospitals
- In our direct markets including the U.S., we increased our customer accounts from 470 to 638
- Increased staff worldwide from 152 to 218
- Enrolled 64 patients into the U.S. BARDA pivotal burns study (53%)
- Enrolled 25 patients into the U.S. DFU Chronic Wound study for health insurance reimbursement (18%)
- Enrolled 35 patients into the chronic wound study with Flinders University South Australia (55%)
- Leased an adjacent property in Port Melbourne to significantly increase manufacturing capacity
- Awarded Victorian Government grant for manufacturing Diabetic Foot Ulcer product (NovoSorb SynPath)
My Analysis
With a strong cash position post the capital raising late last year, $PNV are making good on their investment in sales and marking to accelerate sales.
So, the 43% expansion in headcount (surely, mostly in sales and marketing) is hardly unexpected, and is their fastest expansion for a while. And given that we know it takes up to a year for a new sales and marketing employee to break even, such a scale of investment comes at a necessary cost.
Cash is still being burned. A total of -$8.3m (including leases) which is up from -$2.4m in FY22 (where I am excluding the benfit of the sale of the Melbourne property). With $47m in the bank, the rate of burn is not a concern, although the major spend in the new manufacturing facilities is still to ramp up – so we should expect to see significantly more capital investment in FY24.
Global hospital accounts at 638 are up 35%, and of course new accounts are a leading indicator of the sales growth to follow.
In both the report and the presentation, prominence if being given to the number and breadth of publications demonstrating the expanding clinical applications physicians are finding. And those of us on the DW mailing list have seen the benefits being report. So, another leading indicator of future growth.
The presentation is light on detail in terms of market progress. We can see that US Novosorb sales grew 44.6% (although only 34.0% in USD) and RoW sales reached $13.4m up 133.9%.
On sales, the US constant currency rate is slower that I expected, so it will interesting to hear how this is characterised on the call this afternoon. (There is no evidence of a boost from Integra’s recall of Surgimend.) With US accounts expanding by 58% to 299 from 189, and existing accounts expected to grow sales, the US sales growth number is a bit soft in my view.
RoW is starting to become more material, and this is a key number to watch in the future. Several of these markets including Canada, Hong Kong and India only started during FY23, with several only really getting going in H2. Of course, this is where a lot of the sales and marketing expansion has been, so it is not surprising.
I’ll leave it at that ahead of the call.
My Key Takeaways
Overall, there is something in the result for every thesis. Bears more focused on the short term will point to increased cash burn, rising costs and slowing US Sales. The bulls will point to expanding market footprint with strong RoW becoming material, new accounts, top line growth, and growing positive clinical evidence.
I remain in the bull camp based on this report. But I can see a stronger focus on revenue growth pushing the cash generation profile backwards, and so there is nothing in this report for me to upgrade my valuation. If anything, the upper part of my range is scaling back. But that’s for later in the year.
Based on this result, I am a solid hold. But I want to learn more about US progress, as I am sure others on the call will too.
Disc: Held in RL (4.3%) and SM
Latest email from DW. (I chuckled at his remark about the "Britishness" of the article. Those of us who have experienced David's flamboyant approach to communication will get the joke. But I think that is also a difference between investor relations and publishing in a serious peer-reviewed journal.)
Key Take-away: Growing evidence supporting breadth of potential in BTM in complex wound care.
Side question to StrawMedics: Does anyone know why BTM isn't FDA-approved for full thickness burns, whereas it is approved for this indication in so many other jurisdictions?
----------------------------------------------------
EMAIL from DW follows
Kidd T, Kolaityte V, Bajaj K, Wallace D, Izadi D, Bechar J.
The use of NovoSorb biodegradable temporising matrix in wound management: a literature review and case series.
Journal of Wound Care. 2023; 32(8):470–78.
https://doi.org/10.12968/jowc.2023.32.8.470
This is a retrospective observational case series evaluating the use of NovoSorb BTM in 37 patients across a wide range of wound types including acute trauma, hard-to-heal (chronic) wounds, acute infections, and skin cancer excision.
Data reviewed included patient demographics, wound characteristics, time to BTM integration, time to skin grafting, and the incidence of complications.
Successful outcomes were achieved in most cases, despite the type of wound bed (muscle fascia, exposed tendons/paratenon, bone/periosteum), patient age, and wound size. Success was demonstrated in difficult cases where other treatment options are limited.
The flexibility that BTM provides to treatment pathways is highlighted, enabling factors such as operating theatre availability and patient compliance to be accommodated.
Of the complications arising, the majority were attributed to patient factors that worsen a patient’s healing capacity (e.g. diabetes and peripheral vascular disease). Of note, the authors state that the method used to document complications was over-cautious because a patient with infection and complete skin graft integration was still included, despite a successful clinical outcome.
Valuable learnings regarding the use BTM are included, such as the need for judicious debridement before application, regular wound reviews after application, and the use of wound swabs and appropriate antibiotics.
The authors conclude:
“BTM continues to show promise as an additional tool in the reconstructive ladder, especially in wound beds less amenable to immediate grafting or soft tissue reconstruction.
Additionally, it plays a role in patients not suitable for autologous reconstruction due to fragility, comorbidities or anaesthetic risk for long procedures.
…BTM is robust and complications, such as infection, can often be strategically managed with judicious care and still lead to positive outcomes.”
Disc: Held in RL(5%) and SM
As expected in our speculations yesterday.
PolyNovo is pleased to announce a record monthly sales result of $7.2m (unaudited) for May.
Their Highlights:
• The U.S. had record monthly sales (unaudited) of $A5.2m up 97.3% on STLY
• The rest of the world had record monthly sales (unaudited) of $A1.9m up 189.3% on STLY including encouragingly strong sales in Canada, Hong Kong, and India and a first-time order from the Middle East.
• Total revenue (unaudited) including BARDA for 11 months to 31 May 2023 is $A59.1m up 54.5% on STLY of $A38.3m.
My Take Aways
- Good news, however, it is in the share price earlier this year, so perhaps reverses some of the recent decline, which on a fundamental basis can be argued to be due to softer Jan and Feb (i.e., ignoring shorts action,which has been on the rise again, increasing supply of shares onto the market.)
- Its already in my valuation
- If they finish with a strong June, then the annual result should be ahead of consensus IF April wasn't soft.
- Doing a STLY comparison for a month is ridiculous, given the month-to-month variations - so beware not to pay attention to that on a longer timeframe read across. (Really DW, its shameless pumping!)
- Good to see that new territories are contributing, even if the $ value will be very small
- Overall, good news, but I still wish $PNV could get to just reporting on a regular basis. On average, for a high growth company, every month should be a record month! (OK, that's my irritation scratched.)
Disc: Held in RL and SM
The short email below is just in from DW.
While a competitor product recall can be a huge tailwind to any healthcare firm (witness the tailwind $RMD and $FPH have received over two years from the Phillips recall), it should also serve to remind investors of this key risk in healthcare.
$PNV is a young company, early in the development of its own manufactuing capability. I hope whatever lessons are to be learned from Integra in the fullness of time are learned at $PNV, and that the assistance of any potential tailwind arising, does not lead to complacency.
Turning to the recall, the size of the recall time window indicates that this is something fundamental with the manufacturing process or the product itself. I spent 5 years early in my career as a pharmaceutical operations manager. The spectre of this kind of event is the stuff of nightmares. I wish them well.
Email from David Williams
Integra was down overnight in the US on news that they have “initiated a voluntary global recall of all products manufactured in its Boston, Massachusetts facility distributed between March 1, 2018 and May 22, 2023.”
The greatest impact of the recall is on Tissue Technologies Segment with the voluntary recall including the SurgiMend , PriMatrix , Revize™ and TissueMend™ products. See attached.
I'm not sure all holders here are on the DW mail list. So I am sharing the following which has just come through.
It is yet another example of how surgeons are driving innovation using Novosorb and BTM. We heard David talking about other instances in the last SM meeting.
The article can be accessed via the link ... warning: contains images not for the faint hearted!
While I am online, I attended the $AVH Q1 call this morning. Q1 pcp revenue growth was 40%. This confirms that $PNV is growing the fastest in the "peer group". (Note that $AVH is not strictly a competitor, as ReCell is sometimes used in conjunction with BTM.) I expect to see strong growth numbers in future periods from $AVH, as they have expanded their field sales organisation from 30 to 69 during the period and have received furhter FDA approvals for various indications.)
=======================================
A new BTM article has just been published by Dr Srinjoy Saha from the Apollo Multispecialty Hospital, Kolkata, India.
- Saha S.
Tissue-engineered minimalistic reconstruction of a severely crushed fingertip.
Journal of Stem Cells and Regenerative Medicine. 2023; 19(1):13–17.
https://doi.org/10.46582/jsrm.1901003
This article reports the clinical treatment and outcomes for a patient who presented with a severely crushed right-dominant ring finger following an industrial accident.
The treatment involved the off-label use of NovoSorb foam layers, as well as the use of NovoSorb BTM.
The palmar soft-tissues and fingertip of this patient were destroyed completely, leaving the remaining bone and finger joint exposed. The nail plate was also avulsed from the traumatised underlying nail bed.
To develop new granulations over exposed bone, platelet-rich fibrin (PRF) injections were prepared and injected into the wound. NovoSorb BTM was applied and delaminated after 6 weeks revealing good restoration of soft-tissue volume over the sides.
The main soft-tissue bulk that forms the pulp of the finger was absent. Instead, the bone was covered by only a thin epithelialized layer. To provide additional fingertip volume, the surgeon removed the sealing membrane from the NovoSorb BTM and applied two layers of NovoSorb foam over the thin layer of soft tissue. Finally, standard NovoSorb BTM was applied on the palmar aspect of the finger and the nail bed.
Clinical review at 6 months showed that the regenerated finger appeared mostly identical to the opposite side. It was fully functional and looked a lot like the opposite finger, with well- developed nails. However, due to the loss of bony length caused by the initial trauma, the tip was shortened. The patient
performed all normal activities successfully. In addition, the regenerated finger pulp enabled him to type normally on any computer.
This article is for shareholder interest only as the use is off label.
Regards,
David Williams
Level 29, 55 Collins Street, Melbourne, VIC 3000
T + 61 3 9246 4203 M + 61 414 383 593
E [email protected] www.kidder.com.au
A great meeting with DW. It was great to get the reflections and anecdotes across all aspects of the business - so well done to @Strawman for leading a great discussion and drawing everything out of David that we might have hoped to achieve.
Some of my reflections follow.
Competitive Position
DW sounded even more confident that synthetics are winning over biologics. This is supported by the now slower growth at Integra (where the wound division has annual sales of c. $400m), however, Arora is still growing well so the category overall is expanding. (I know there are nuances in the specific applications to different indications, which mean these products are not always going head-to-head.) Ideally, companies like Integra and Aroa - who also have large salesforces - continue to expand the overall market. Then $PNV can ultimately mop it all up. That's the super-bull case, by the way!
R&D
New products (hernia, breast, drug elution) will be over a longer timeframe. PNV used to talk about timeframes, but since the new head of R&D (and Swami) have come onboard, they have stopped doing this. I seem to recall the Head of R&D makeing unscripted remarks at the last AGM where he referred to hernia and breast requiring different mechanical properties. I suspect this means back to the drawing board for the polymer scientists and also there was a reference to need to reach out and network with leading researchers in the field. So I am very much seeing hernia, breast and drug elution as long term "blue sky", which is consistent with $PNV dropping timeframes in their communications.
On capital allocation, it will be interesting to see in the annual results how much is going into R&D. Hopefully, this can be managed in a sustainable fashion as a certain % of revenue.
I'm not concerned by this by the vague timeline for future developments. As David's anecdotes make clear there is huge opportunity for widening the dermal repair applications for BTM and MTX, and progressing the global roll-out. If there can really ramp this up over the next 3-5 years, then $PNV will also have the cashflow to self-fund future developments.
Share Price Action
For what it's worth, I'm not convinced by David's explanation about the SP action being fully-explained by shorts. Shorts are at 2.68% (2-May), vs. their peak of 11.4% mid-last year, and the flow of shares into the market from short selling has been 9,000,000 since the low point in 16-March. With about 30 trading days, that's 300,000 on average per day vs a typical daily volume of c. 2 million.
I think four factors in combination have got us to where we are today:
- David's sale in March
- The market knows they announce the $xm revenue months, and it took a while getting to $6m
- The trading update was a weaker sales number than many bulls (including me) had for their FY23 revenue forecasts
- And yes, 9,000,000 of shorts makes a contribution in the supply-demand balance.
None of this worries me at ths stage. Some quarterly periods will be stronger and some will be weaker. Based on established SP volatility, $PNV is clearly still a trader's stock, so its going to contine to be a volatile ride. But, if you are a long term investor, now could present a great opportunity.
Valuation
Overall, today's meeting leaves me unchanged on conviction (i.e. very strong). I'll review my valuation cases once the FY is in later in the year.
In terms of analysts, 4 of 6 reports on www.marketscreener.com have reduced SP since the recent updates, so the average target SP has gone from $2.53 (close to my central case) to $2.25.
However, this warrants a further look. because one valuation has gone from at or above $1.90 to $0.90. In my view there is nothing in the recent disclosures that makes me think that is plausible (even my Bear case is $1.63). So I wonder if any StrawPeople have seen this report? Ignoring this oddball result, the target has nudged down only very slightly.
For now, I'm happy for the team at $PNV to crack with their great work!
Disc: Held IRL and SM
NovoSorb ® MTX First Sales and March 2023 $6m Revenue
Their headlines
- Monthly Revenue (unaudited) including BARDA of A$6.4m in March, up 48.2% pcp, including record BTM sales of A$5,45m.
- Sales Revenue (unaudited) for 9 months to 31 March 2023 of A$41.1M up 49.8% pcp.
- Total Revenue (unaudited) for 9 months to 31 March of $A45.2m, up 47.8% on pcp $30.6m
My Observations
Continued good progress, even if tracking well below my model which has SP target of $2.46, vs. $1.63 opening today.
3rd Q revenue was ($45.2-$30.6)=$14.6m, which means if March was $6.4m, then Jan and Feb averaged $4.1m, which gives an indication of the month to month variability. "Lumpy" as DW comments.
In my previous straw I noted the recent results of $ARX and $IART (NASDAQ) and it is clear that - assuming most of the growth continues to be in USA - that $PNV is growing more strongly than both, and much more strongly than $IART, the incumbent.
So even if the rate of growth is moderating (from >60% to c. 50%) it is clear that $PNV is taking a larger share of the market - a good sign.
No real details on India, other than by omission if new hospitals have been added in USA, Canada and HK, then no new hospitals have been added in India, or elsewhere for that matter.
Initial market SP response a modest rise.
My Key Takeaway
An OK result, but in the absence or further news before the FY, I don't think it is strong enough to significantly reverse the SP decline we've seen in recent weeks, but it should be enough to stabilise it.
Disc: Held IRL and SM
Now sure if all holders are on the David Williams email list. So here is news of a publication from a Brisbane surgeon reviewing pediatric use of BTM. What I find interesting is the breadth of clinical application (which presumably reads across to potential applications in the adult market). Thermal burns being less than a third of applications.
(Good on David - he might have just purchased a house in the US, but he is still busy spending his weekend time promoting $PNV)
Email Full Text (link to paper embedded)
I am pleased to share this recent article reporting the outcomes of paediatric BTM case series from the team led by Professor Roy Kimble at the Queensland Children’s Hospital, Brisbane, Australia.
Professor Kimble has vast surgical experience with BTM and has shared much of this in various conferences and peer-to-peer sessions around the world. This article brings those insights together in a comprehensive way.
Storey K, Lalloz M, Choy K-T, McBride CA, McMillan C, Das Gupta R, Patel B, Choo K, Stefanutti G, Borzi P, Phua Y, Bade S, Griffin B, Kimble RM.
The versatility of Biodegradable Temporising Matrix – A 63 paediatric case series with complex wounds.
Burns Open. 2023; 20 March [in press].
https://doi.org/10.1016/j.burnso.2023.03.002
A retrospective database review was conducted and identified all patients in whom BTM was applied between September 2018 to June 2022.
Data collected included demographics, type of injury, description of defect, and time frame from BTM application to SSG. Complications were also collated along with subsequent scar management strategies.
The Brisbane Burn Scar Impact Profile (BBSIP) was used to assess health related outcomes and was used to determine the appropriate mode of scar management patients received.
Over a four-year period, 63 children received BTM for wound closure. Patient age range from 10 months to 14 years.
The mechanisms of injury or original pathology included:
- thermal burns (N=19; contact, flame, scald).
- friction (N=10; treadmill, bike/motorbike wheel, other)
- vascular malformation (N=7)
- scar reconstruction (N=6)
- non-infective skin ulcer/defect (N=5)
- electrical (N=3)
- degloving (N=3)
- fasciotomy defect (N=3)
- dog bite (N=2)
- infective skin necrosis (N=2)
- melanocytic naevus (N=2)
- dermatofibrosarcoma protuberans (N=1)
Methods of securing BTM were clinician dependant and included staples or absorbable monofilament sutures. Histoacryl was used on small areas of the face, fingers, and feet and often in combination with sutures.
Mepitel under Acticoat was the most used primary dressings over both BTM and SSG in all but 8 children. Most secondary dressings involved use of NPWT. If no NPWT was applied, Kerlix or Melolin were the most common secondary dressings applied.
Scar management was similar in all children. Initially, this involved the use of silicone gel and/or pressure garments depending on the size and location of the injury. Further scar treatment including microneedling, and laser therapy (CO2 or pulsed dye) were used on 21 children. Surgical reconstruction with Z-plasty and full thickness skin grafts (FTSG), or serial excision was performed on 8 children.
Of the 29 patients where the BBSIP assessment was completed as part of their scar management treatment, the majority reported little to no concern when looking at the impact, itch, appearance, friendship, and emotional stress that the scar causes within their life.
Detailed outcomes for the various indications are included in the paper.
The Discussion provides further insights on the following concepts and topics:
- BTM buys you time
- BTM converts complex wounds to smaller ones
- The use of NPWT
- If not secured with staples or sutures, ensure an experienced clinician removes dressings
- Wound colonisation is still compatible with a good result
- Split thickness skin grafts are not the only type to use
- BTM does not negate need for scar prevention
- BTM impacts the patient and their family
The authors conclude:
“Our experience with BTM in a variety of complex wounds has shown it to be a viable option to provide early wound closure for improved long-term patient outcomes, that is able to withstand bacterial colonisation. While long-term scar outcomes have only been observed over the past four years, patient, parent, and clinician observation have proved promising.
From our first application to our current treatment, the lessons we have learnt in using this product continues to guide us to ensure the best results for our paediatric patients and families.
BTM from our account has proven to be a more durable and versatile product, compared to previously used dermal matrices, with promising prospects for future management of complex paediatric wounds.“
David Williams
Chairman
ASX Code: PNV
M +61 414 383 593
E [email protected].au
David's buying a house in the US and has offloaded 4.75m shares. Ouch!
My first thought is, of all his potential sources of capital, given that he offloaded $PNV, what does that say about his view of the near-term propsects of the company's progression?
Disc: Held in RL and SM
FULL TEXT
Chairman Mr David Williams has sold 4.75m shares, the proceeds of which will part settle a US property purchase. Mr Williams still holds 21,384,432 fully paid ordinary shares and does not intend to sell more shares for the foreseeable future. This announcement has been authorised by PolyNovo Company Secretary Jan-Marcel Gielen.
The purpose of this straw is to review the progress of $PNV in the context of its sector competitors. It contains some analysis of possible market evolution. Although somewhat academic, it may be of interest to holders of $PNV, $AVH and $ARX, so I’ve decided to publish it. (Sorry its such a long one, but I do this kind of analysis on my top holdings and I don't ahve the appetite to edit it down.)
I have done the work to further my own understanding of the long-term growth assumptions in my $PNV valuation ahead of updating my company valuation later this year.
Peer Group
The companies in the sector considered are (the “Peer Group”): Aroa (ARX), Avita (AVH), and Integra (IART).
It is important to understand that across the Peer Group there is a wide range of products with a wide range of clinical applications including burns and complex wounds. These products are not today always in direct competition with each other. For example, $PNV’s BTM can be used for dermal repair in conjunction with $AVH’s Recell, which can be sprayed on top. In this respect they are complementary products. However, each of the companies is continually trying to extend its product portfolio and applications. For example, $ARX is used in complex wounds, which is a key area $PNV is trying to grow into from its existing base in burns. As of last year the CEO of $ARX said he did not consider $PNV a competitor. But I think that will change (is changing)!
Sales Growth
Table 1 below shows the revenue growth of revenue during calendar year 2022.
Table 1: Peer Group Revenue Growth 2021 to 2022

Source: Company Presentations and Accounts
Notes:
1) Because of the $ARX reporting calendar, I have used growth in cash receipts as a proxy and triangulated this with its target revenue growth for FY23 ending in April 2023.
2) $IART has a more complex portfolio of products. Fortunately, in segment reporting it breaks out sales for its “Total Tissue Technologies” segment. So, I have used this.
Observations from the table
$PNV: Is easily the fastest growing in the Peer Group. In addition, we are yet to see the impact of the accelerated ramp-up in sales and marketing personnel and territory expansions following the December capital raise.
$AVH: announced that they expect FDA approval for Soft Tissue and Vitiligo applications of Recell in June 2023 and to be ready to launch on 1-July. They have identified the Vitiligo market to have a patient population of 5 x Burns and Soft tissue repair (see Figure 2 below). As a result, they have announced plans to increase their US field sales organisation from 30 to 70 professionals. While their sales growth looks modest at 4%, this is masked by the large $8m BARDA payment in 2021. Excluding BARDA, commercial sales grew by 35%. In FY2023 they have given guidance of $49-$51m, which would represent 45% growth over 2022 at the midpoint. These factors have no doubt driven the recovery in share price over recent months. There is, therefore, the potential for very material growth for $AVH in a separate market in which other Peer Group members would not compete (Vitiligo).
In December, $IART acquired Strategic Innovation Associates (SIA), makers of Durasorb, an innovative resorbable synthetic mesh, for use in breast reconstruction. This complements the existing product Surgimed (a biologic) in Hernia repair and plastic and reconstructive surgery. CEO Jan de Witte said “Now with DuraSorb and SurgiMend, we have a path to securing the first and second PMA products in the market. And by offering 2 distinct product solutions to plastic and reconstructive surgeons, Integra can build a leading position by addressing various clinical, contracting, and economic needs across different sites of care.” From my previous $PNV straw, I noted that CEO Swami Raote has been more circumspect on the development timeline and certainty for the development of breast and hernia products. So, it appears that once again $PNV will be following a “next-to-market” path in the years ahead, with a synthetic mesh already established in breast reconstruction and potentially a biologic in hernia repair. It is further interesting to note that $IART now has a synthetic in its portfolio.
Market Size
I found the following slide (Figure 1) from the recent $AVH investor. It is interesting to note that $PNV performed about 8,000 procedures in 1H FY23, an annual run rate of 16,000. With 84% of commercial sales in USA, that means about 13,500 procedures were carried out in the USA. Given an estimated market size for the USA of 145,000, that points to $PNV having a market share of 9% (Note: this significantly greater than the number estimated below based on revenue share).
Figure 1: US Market

Source: $AVH Company Presentation 2023
Separately, the global TAM for surgical reconstruction and complex wound care is estimated by $IART to be $2bn and growing at 5-7% p.a. On a sales basis, the Peer Group total sales account for only 30% of the market, with $IART at 27% market share and $PNV at <3%. There are two reasons for this: 1) for many indications, including burns, the standard of care remains autografts or skins graft, which do not involve a dermal implant product and 2) there are other products produced by other companies not addressed in this analysis.
While the estimates for $PNV’s market share are very different based on number of procedures and market value methods, both are consistent in supporting what we know in that its market share is small (3-10%) and growing much faster (c 60% p.a.) than they overall market, which is currently adding c. $100-$150m revenue opportunity p.a. - much more that $PNV's current total sales.
Part of the discrepancy between the methods lies in that the procedure count method is focused on the US market, whereas the TAM analysis is global. Clearly, all members in the Peer Group are highly-focused on the US Market. However, while the largest market, it only accounts for 43% of the global medical devices market. Western Europe is 24% and Japan is 7%. Current revenues of all Peer Group members are highly US-focused (70%-90%).
Growth Scenario & Assumptions
The basic question I am interested to understand is how long and how strong can $PNV revenue growth be in a competitive context? i.e., are the current growth assumptions in my valuation meaningful in the context of the overall market size, growth, and evolution of competitors?
I have modelled several scenarios, and (for illustration) here present only one.
2022 market size is US$2bn, with revenue plugged in for $PNV, $AVH and $ARX aggregated, $IART as the market leader shown separately.
Because there are several other products in the market (e.g., competing with $AVH’s spray on skin cell solution) and other implants, although I haven’t identified and quantified these, I have put in a placeholder for the current market value of $50m growing at a steady 20% per annum. (I would update this should in the future I gain more market intelligence.)
The balance is assumed to be “Other Care Systems”. This includes the autograft (skin grafts) which is the current standard of care for many burns and complex wounds.
The overall market grows at 6% p.a., and IART grows at 7% p.a., in line with its corporate assumptions.
In a “Bull Case”, $PNV starts off growing at 50% p.a., with growth rate declining by 1.5% p.a., and the Peer Group Minors starting at 35% p.a. growth, declining at 1% p.a.
Figure 2 (below) shows the market evolution over the 10-year period for this scenario. Key metrics for 2022 and 2031 have been extracted from the chart in Table 2 (below).
Figure 2

Source: Simulation
Table 2

Source: Simulation Analysis
Of course, you can create any market evolution scenario you like, depending on the assumptions. However, this analysis has yielded the following insights.
- If $IART continues to grow in line with corporate goals, they will still be the market leader in 2031, with a market share of 29%.
- If $PNV continues to grow at 50% pa in 2023, with the rate of growth declining by 1.5% per annum to reach a growth rate of 38% in 2031, it will by that time almost match the sales of $IART ((US$981m vs $989m). Despite its success, in this scenario, given the head start of $IART, even if it sustains a decade of high growth it will still be #2.
- There is of course a potential SUPER BULL case, wherein synthetics are proven superior to biologics via comparison clinical trials. In this case, it would be conceivable that $PNV could take further share from $IART and $ARX. I have not modelled that.
- Over the coming years, as both the current market leader $IART and $PNV seek to grow, the existing market and its underlying growth are sufficient to accommodate both companies achieving almost $1bn annual sales by 2031, provided that there is a continual transition from the current standard of care to these therapies and that $IART and $PNV prove long term preferred solutions ahead of $ARX and $AVH and that no new disruptive technology emerges. (Continuing to scan the market will give early warning of this, in just the same way that $IART can see $PNV coming after it.)
- By 2031, if $IART and $PNV both succeed in achieving these results, the combined market share of these market leaders will be 58%, with room for $ARX and $AVH to have 10% market share each and 22% for others. This is not an unreasonable competitive market structure in healthcare, which space for 4 successful, profitable therapies.
- The revenue growth assumptions in this simulation ARE SIGNIFICANTLY HIGHER than the growth assumptions in both my current BEAR ($1.63/share) case and BULL ($3.28/share) case valuation scenarios.
- Latest period $PNV revenue growth of 62% (which does not include the impact of accelerated investments in sales and marketing) is significantly ahead of the simulation starting point of 50%. Provided at FY $PNV can sustain or even accelerate revenue growth beyond 62%, then a significant upgrade to the BULL CASE appears to be justified.
Conclusions
Simulation models and long-term projections can result in GIGO (Garbage-In-Garbage-Out). However, models can also provide valuable insights.
This modelling confirms that there is a justifiable BULL case for $PNV that significantly exceeds my current BULL Case valuation where the 2031 revenue growth rate is 19.0%, vs. 38% is the simulation presented here.
Such a BULL Case would lead to a valuation significantly greater than $3.28/share. Provided growth and margins results are confirmed at the FY23 results later this year, I expect a major upgrade will result in my next DCF update.
Doing this competitor analysis has provided me with valuable markers to track $PNVs sector peers, which in turn will continue to inform my valuation of $PNV.
Reality Check – Current Valuations
Of the peer group, only $IART is today a consistently profitable, growing business. Of the three small companies, $AVH and $PNV are yet break and $ARX has just broken even.
While clearly the strongest growing of the Peer Group, a lot of $PNV’s success is included in its lofty revenue multiple. So its share price will continue to respond in a volatile manner with each readout on revenues and costs, until it develops a consistent earnings trajectory – some years away. Shareholders will need to have an ongoing stomach for volatility, but my analysis indicates that the potential rewards are there.
It also follows that, because several analysts publish research based on available multiples, there will be occasions over the coming years when “Sell” recommendations are issue based on sector comparisons. We’ve already seen this last year. In addition, traders will trade. My approach will, however, cotinue to keep an eye on the long-term prize.
Table 3: Current Peer Group Valuations

Source: www.marketscreener.com 2023 consensus forecasts
Disc:
$PNV: Held IRL (7.2%) and SM (25%)
$ARX: Recently exited IRL and SM, to reallocate capital and manage portfolio exposure to this sector.
$AVH: Not Held; Watching
$IART: Not held
There was nothing material from this afternoon's call to share. However, there are some insights that I found helpful.
US
- Strong momentum sustaining
- Question on Integra feeling the heat and cutting prices. DW batted this away.
Europe
- David Williams (DW) reported that both UK/Ire and Germany (Ger/Aus/Swi/Belg) are starting to get "real traction", after slow starts over the last two years. "They'll soon be bigger than ANZ."
India
- Selling hasn't started yet as there are various administrative items being finalised ("next few weeks")
- 3 Centres of Excellence have signed up; 25 more identified. These will be key to training surgeons
- S&M staff of 20 identified covering the major geogrpaphic areas; plus 2 senior leaders
- India will be used to enrol patients in the US BARDA trial, thereby accelerating its outcome
- Surgeon trials have started with good results so far
- India is "pure market development" - the biologics aren't there
- Pricing will be "market appropriate" (Swami knows what he is doing from years in medical devices in developing and developed markets with J&J. Margins are huge, so they have room to move.)
ROW Overall
- Consequently, should see continued strong ROW growth (currently >100% p.a.) from an increasing base
Employee Expenses
- Due to headcount ramp-up, FY employee expense expected to be just over 100% of PCP
- This excludes the exceptional share based costs from FY22 (referred to in the slides and my earlier straw)
DFU (Diabetic Foot Ulcer) Trial (Last ASX announcement)
- Suspension of enrolment is needed to make sure that the study protocol correctly maps to the standard of care
- It sounds like this is an intervention to tweak the protocol to make sure the results are valid. (To me it sounds like a case of a "stitch in time saves nine.")
Broadening Clinincal Applications for BTM
- Swami's key focus is how to figure out the right way to take BTM to other places in the hospital
- Individual surgeons are innovating, (e.g., BTM being use to save limbs in trauma treatment)
- But this then has to be turned into a model that the salesforce can disseminate
- Trauma and reconstruction are big future categories (but it will be a longer burn)
R&D: Hernia and Breast
- Swami is working to ensure that $PNV accesses the right development experts (outside the company)
- He remains convinced that both hernia and breast applications will emerge, but "R&D is never and slam-dunk, so we can't say for certain"
- I believe we are now starting to get some realism into the discussion on developments. I never trusted the earlier notional timelines, and this is yet another benefit of getting a seasoned industry executive in place
Capex
- Likely to be c. $1m for full year
- More in FY24 and FY25 for the new manufacturing units which expand 2-shift capacity from $120m annual sales to $500m
- Details per capital raise documents
Cash Flow
- Expect to be close to breakeven for the FY; however, management are strongly prioritising growth and will continue to
Closing Remarks (DW)
- We did 10,000 patients in FY22
- We did 8,000 patient in the first half of FY23
- We will eventally be talking about "hundreds of thousands of patients" (SR nodding)
- "We've only just begun" (cue The Carpenters, slow fade)
Overall Impressions
- David, Swami and Jan all appeared to work well together
- This was a well organised, well executed presentation, without descending into endless adhoc anecdotes and answering every granular question
- 15-20 minutes of presentation and a solid 40 minutes on Q&A
- Great job team. I'm so glad $PNV has a CEO again.
Valuation
- I will wait to update my model until the FY.
- With the acceleration investment, I need to see more data on revenue and costs over time
- From a quick check, nothing is out of whack. So happy with $2.46 for now.
Disc: Held
$PNV today released 1H FY23 Results ahead of the afternoon's analyst call. Note that top-line revenue numbers have been pre-released. As usual, this straw includes their highlights, my analysis and key takeaways.
Their Highlights
PolyNovo’s ASX release on 16 January 2023 pointed to record sales growth of 67.5%.
The half year audited results attached to this release show:
• Record 1H FY23 sales of A$27.3m up 67.5% on STLY of A$16.3m
• Total revenue including BARDA of A$29.5m up 62.2% on STLY of A$18.2m
• Strong growth in U.S. achieving 1H FY23 record sales of A$22.8m up 61.0% vs. STLY of A$14.1m
• ROW sales of A$4.5m up by 110.1% vs. STLY A$2.1m including new sales in Hong Kong and Canada.
• The Group recorded a net loss after tax of A$3.8m (1H FY22: A$1.6m profit). The profit in 1H FY22 included the reversal of A$4.7m in share awards and share options expense forfeited by the previous CEO and COO on their resignations and an unrealised forex gain of A$0.4m. Excluding these items results in an underlying loss of A$2.5m for the prior period.
During the Period, the Company’s other key initiatives and achievements include:
• A $53,000,000 capital raising
• First $5 million BTM sales month in September (Sep 2022: $5,402,454) recurring in October ($5,263,100) and December ($5,306,540)
• Appointment of CEO Swami Raote
• Received FDA 510(k) clearance for NovoSorb MTX
• Entered Hong Kong, India, and Canada markets in December
• Leased the adjacent property in Port Melbourne to significantly increase manufacturing capacity
• Commenced SynPath Diabetic Foot Ulcer Clinical Trial
• Awarded Victorian Government grant for manufacturing Diabetic Foot Ulcer product (SynPath)
• Increasing sales teams and customer base globally
• Produced for 1H FY23 the equivalent of 74% of the total devices produced in FY22
Analysis
Revenue
On revenue, these were pre-released, so there was no material new information. A breakdown of the monthly sales following the first $5m month in September, shows that both October and December exceeded $5m.
In terms of revenue drivers, Figure 1 (Slide 5) gives a good overview. Growth in Customers (Hospitals) and Employees (a large portion being sales and marketing) are leading indicators of future revenue growth, as it takes time for the sales to grow once both a new account opens and a new salesperson is in place. This should give confidence of a strong trajectory through H2. The slide makes the point that a return to >60% revenue growth marks the return to better customer access post-COVID.
Figure 1 (Slide 5)

Figure 2 (Slide 6)

On Figure 2 (above), I see that ANZ and RoW are now starting to become significant. At $4.6m in aggregate, they are about the same as the USA was in 1H FY19. While ANZ is relatively mature, it is still growing at >60% and we are yet to see meaningful contributions from Canada, Hong Kong and India. Interestingly, there is no mention of Europe beyond UK. So RoW should be expected to maintain strong growth as we start to get early traction in new territories. BARDA is also growing, so I wonder how far off we are from seeing a strategic stocking decision?
Costs
Today is all about understanding how expensive the cost of accelerated expansion is going to be.
Comparing 1H23 to 1H22, the incremental revenue was $11.2m, while incremental costs added are $16.9m. That sounds pretty bad on the face of it. However, the previous period included a credit of $4.7m reversing lapsed options of departing executives, so the underlying incremental cost is only $12.2m, by my calculation. While I am not a fan of underyling corrections - particularly when they happen every period - in this case, I hope we will see some management stability and so I'll let that adjustment pass. Overall, cost increases will always run ahead of revenue growth. So, the real test of this strategy is going to be in the FY23 and 1H FY24 reports.
Of course, it is important to remember that we haven’t seen the full effect of the step-up in cost base as the changes in increased staffing occurred through the half. We’ll see the full effect in the H2 numbers at FY. I expect to hear some questions on this from the analysts on this afternoon’s call about a forward view on cost evolution.
Profit and Cash Flow
Unexpectedly, therefore, we’ve seen a small increase in losses to $(2.2)m.
FCF was $(3.1)m compared with $(3.7)m in the PCP (note: I'm deducting lease costs from the FinCF line from OpCF), so in the context of now having $50m in cash available, the cash burn is sustainable. Of course, in FY24 and FY25 we are going to see a step-up in capex, as new facilities are built. However, if revenue continues to grow at current rates, the cash generation should mean that cash reserves are not eaten into that much.
My Takeaways
With revenue numbers pre-released, and the capital raise clearly indicating that SR had moved DW on from the “boot-strapping” strategy (no doubt a condition of SR’s joining), today was always going to be about how much costs are stepping up to drive expansion. Because of timing, we are only getting a first view of that.
Overall, it’s a bare-bones, no-nonsense presentation, which I personally find refreshing. It will be interesting to see how it is received. The analysts have been used to getting more granular detail, so I expect there will be more digging in the Q&A, as analysts try to get their FY numbers right. (I’m looking forward to the tussle!)
I am happy with the progress shown in this report. Nothing has a material bearing on my valuation, which sits at $2.46 (being the average of distinct bull and bear case DCF scenarios.) I will wait to do an industry read across including Aroa, Integra and Avita reports to see if this offer any insights on competitive positioning over the coming weeks.
Disc: Held IRL ( 6.8%) and SM (22.9%)
Those following $PNV will be aware that as part of the December capital raise, there was a $3m component of Director contributions. We are all used to DW buying shares in the company and, at the time, I assumed he would be putting his hand in his wallet again.
However, today we got to see who bought what. (Personally, I don't know if this detail was disclosed at the EGM, as I wasn't paying attention. Anyway it is news to me.)
- David Williams $2.229m
- Bruce Rathie $0.380m
- Andrew Lumsden $0.095m
- Christine Emmanuek $0.266m
- Robyn Elliot $0.029m
David aside, these are significant increases in the personal holdings of several of the directors, which I take as encouraging. I am particularly encouraged because this comes about half a year after Swami has joined as the CEO, so for the Board to be feeling good about the company to this extent indicates to me that internal alignment on strategy and execution is there. It is not a big deal, but a positive sign, I think.
What would put the icing on the cake would be for the CEO to get with the program!
Disc: Held IRL (6.7%) SM (23.5%)
@west has posted $PNVs 1H23 Revenue Report, and the market has opened positively.
If we look at the last six half-yearly periods, $PNV has achieved an average revenue growth rate (H-o-H) of 20% (which is 44% annually).
1H23 is 25% on 2H22, so that's above trend even if below the previous result of 30% (2H22 v 1H22). Of course, the trend includes the drag of COVID impacting hospital access.
If $PNV can sustain 25% in the current half, then it would be on track to acheve total revenue for FY23 of $66m, which is above consensus. Given the gearing up in new markets, this might be exceeded, particularly if US momentum is maintained.
(My valuation has $70m for FY23, which is a bit toppy, but who knows.)
Overall, an in-line result, and looking forward to seeing the full set of numbers in a few weeks.
Disc: Held in RL and SM.
Further to @GazD posting the $PNV presentation to the JP Morgan Healthcare Conference in San Francisco today, while the presentation contains no material new information, it is a good up to date overview of the business with summaries of market focus (including where we can expect movement in the near future) and product portfolio.
Over the last 1-2 weeks we have seen the SP increase by over 30% from the capital raise price of $1.90 on NO NEWS whatsoever.
The SP at writing of $2.48 is bang on my latest valuation of $2.46 from 4 months ago - so I am a clear HOLD, even though it is easily my largest holding in SM (24%) and RL (10%).
A word of warning, however, if H1 results don't exceed lofty expectations, there could be a significant pullback. We have some way to go until $PNV becomes a reliable performer. Being close to breakeven, the actual profit number might be finely poised between continually increasing revenues and cost expansion due to i) expensive seniors hires ii) R&D buildout and iii) the broadening of the sales and marketing footprint. Impossible to call where it will land, so if you are interested in the stock then be prepared for moves either way and don't be surprised by downside volatility. These short term moves are of no real interest to me, as I hold with high conviction for the long term.
I'll review my valuation after the 1H results in a few weeks time.
In addtion, it is worth noting that short interests (see below) have been progressively falling - the reduction over the last 6 months representing a sigificant withdrawal of supply of shares from the market, and going some way to explaining the SP strength.

$PNV has now concluded the capital raising process.
The SPP at $1.90 was oversubscribed, with applications for $35.27m against the initial goal of $17m. The Board have exercised its discretion and will issue $20m of new shares, an additional $3m. So there will be a scale back, but this will be based on retaining existing shareholders pro rata shares.
Overall, $53m has been raised:
- Institutional $30m
- Directors $3m
- SPP $20m
So the firm is now well-capitalised to broaden and deepen sales and marketing footprint, building new facilities in 2023/2024 and drive R&D.
$PNV is my largest holding in SM and RL, so my concentration rules prevented me from buying more. I will continue to watch progress over the next year with interest, particularly in seeing how the sales trajectory moves.
Disc: Held
Details of the $PNV capital raising now out.
also a detailed presentation.
Raise is:
- $30m institutional (+2.4% shares)
- $3.0m Director shares, subject to EGM in January
- SPP up to $17.0m for retail shareholders.
So the raising represents a dilution of <5%.
Once again David is showing total alignment. I assume he will be providing the lion's share of the Director contribution.
Looks like the insitutional bit is done, as it comes out of trading halt tomorrow.
Rationale is about organic acceleration: sales & marketing, R&D, new indications, and production facilities to support $500m sales. (that's more than 5x consensus 2024 sales!)
My conclusion: Good idea. Boot-strapping off the smell of an oily rag would be a drag on growth. Go big or go home.
Details on the rationale (from the release)
Accelerating PolyNovo’s growth ambitions PolyNovo is well placed to achieve significant growth in the near-term with four key vectors driving execution of the growth plan:
• Geographic and sales team expansion: Ongoing growth in core markets, including continued expansion in US presence with 54% increase in US staff over FY23F while simultaneously entering Canada, Hong Kong and India, with revenue expected from CY22. Exploratory steps have been taken to facilitate entry into China and Japan – the global #2 and #3 medical device markets
• New indications for NovoSorb: NovoSorb BTM is already a leader in third degree burns in Australia and on a steep growth curve in US burns. There is a significant opportunity to increase TAM through access to new markets with existing products. Surgeon led insights and innovation are driving PolyNovo to work with clinicians and regulators for new indications and applications
• New products: Investing in R&D capabilities to support new product ranges as well as upstream application and marketing, insight generation, biologic, pre-clinical sciences, process and package engineering. Potential for alliances with global category leaders and academia for Clinical & Health-economics evidence. PolyNovo sees an opportunity to enter orthobiologics, breast reconstruction and fascia repair
• Capacity expansion to satisfy growth: PolyNovo will commence development of a new colocated facility with production, R&D and office facilities. The facility will be designed to support an additional A$500m in revenue a focus on flexibility, modularity and automation. Total expected build cost of A$25m, with spend predominantly incurred in FY24F
Disc: Held in RL and SM
Trading halt and capital raise for $PNV.
Is this to fund more aggressive sales and marketing (witness the cost impact of Swami's push into Asia) or have they found the market access acquisition they've been transparently looking for? (No reference to an acquisitionin the release.)
Timing is hardly surprising given the run-up in share price over recent weeks.
If it is linked to an acquisiton, then I am happy as they know what target they are looking for.
If it is linked to supporting organic S&M and R&D, then I can understand it, too. David has done well to run it off the smell of an oily rag over the last year or two, but there is a big global opportunity they need to capture. It was almost inevitable, in my view, that as Swami started to drive strategy, capital would be needed.
I look forward to hearing more. At 8% of my RW portfolio and >20% of SM, I'll not be participating for more. But I will stay buckled in for the ride.
Disc. Held
$PNV announcing entry into the Indian market. This has been well and truly signalled, and I am not sure why it warrants an ASX announcement, but clearly DW is not one to pass up the opportunity to create a buzz.
In my own modelling, I have low/middle income markets much further down the track.
$PNV tend to be quite granular about where their sales people are in the regular updates and also what sales they are getting. So if they provide this level of transparency about India it will help investors to better understand the ultimate market potential for BTM. So, in that respect this is a positive development, but on its own not yet any basis to update the valuation.
Of course, this is the second move driven by Swami, the first being Kong Kong a couple of weeks ago. So success of these initiatives is key to him making his mark on the company. (I have to say that it is not without risk. as the tried and tested model for pharma and healthcare is USA>>Europe>>Japan>>other rich OCED first, as that is where higher pricing x customers drive value.)
Disc; Held RL (7.5%) SM (22%)
Attended $AGM today. Nothing immediately significant to report in terms of valuation. Conviction reinforced … but recognise the bias of the luv fest.
Some observations.
- Complete alignment in the messages … it’s about sales and marketing … getting it out there
- Canada to be rolled into the established US “cookie cutter” via sales partner (old news)
- US update … it takes 6-8 months on average for a sales rep to start to break even (OMG it’s different every time. My model assumes 12m.). People are now approaching from Intégra and asking to join, and these can break even from the outset because they have relationships and can switch accounts.
- Hong Kong is a bridgehead to China. Next visit in a couple of weeks. Looking to get a couple of people on the ground soon.
- India happening by stealth. If I heard correctly, SR already has two people in place and likely 6-7 by year end. Launch at conference in a few weeks. SR thinks it’s a big deal. If they prove it commercially, then it is a game changer as my model has low/middle income countries years later.
- Hernia R&D … there are some polymer strength issues, so sounds like this might be later … actually good as it keeps laser focus on dermal repair. (Unrehearsed candour from the new head of R&D…in fact the whole event seemed pretty unrehearsed.)
- Still no real story for Europe. France/ Spain next but still searching for the right partner.
- (Valerie and her family had a nice meal out in Hong Kong … sorry, insider joke)
Swami: “We’ve only just begun…” (cue music,… lights fade. Goodnight)
Disc: Held RL and SM
https://newswire.iguana2.com/af5f4d73c1a54a33/pnv.asx/3A605463/PNV_Hong_Kong_Two_cases_Two_surgeons
Australian sales leader heads for a flying visit to assist two surgeons get started in using BTM.
The first sign of the new CEO pushing the S&M strategy. While of itself not a material annoucement, it is worth recognising that the GBA area around Hong Kong has population of 80-90 million.
This indicates a potential Asian strategy of FIFO visits to seed the market, followed by implemeent small teams focusing on the mega-cities, allowing small teams to establish the business model in each country.
Interesting to see how this develops. I expect we'll see India next, as SR has already indicated.
Disc: Held in RL (7.5%) and SM (22%)
Approval for BTM in the next key market - Canada. DW and SR have previously spoken about the sales partnership they've put in place for Canada, with [17?] identified sales reps $PNV are training up. This bodes well for early traction which will be managed out of the USA by the SVP S&M Americas Ed Graubart.
Next stop: AGM this Friday.
Disc: Held RL (7.5%) and SM (22%).
DW has started serial nibbling again. Day 2, increasing his holding by another 0.4% (156k worth). He buys carefully in small nibbles to take up the slack.
If he is still buying at c. $1.70, given that he already holds c. 25m share, then clearly he is of the view that the value is more in line with the Strawman consensus of $2.26. (David, what is your SM handle?) Additionally, he tends to exercise judgement in timing purchases, so I take that as a positive in that he is seeing continued positive momentun running into the AGM at the end of the month.
Note, the broker consensus of $1.69 (n=6, mmarketscreener.com) is dragged down by Jeffries at $1.11 who as pointed out recently by @Rick insist on revenue multiple comparisons, taking no account of either comparables margins or growth rates.
Alas, I won't be following David, as my holding is at maximum portfolio weight (RL 8%; 20% in SM).
A positive update. September achieves first $5m month at $5.4m .
Q1 sales of $12.5m, up 73.3% on PCP, with US growing by 61.3% on a constant currency basis.
There is a currency tailwind in this overall Q1 number.
Good to see US sales growth has picked up again after softer growth in a couple of the Q4 months.
ROW grew by 84.0%.
(Reaching my target FY23 sales of $70m is probably out of reach, but that supports a SP of $2.46 and I am way ahead of consensus which is $63.5m)
Disc: Held IRL and SM
Research update from Bell Potter received with morning from $PNV Investor Relations
TP upgraded significantly from $1.50 to $1.90.
(I had a few helpings yesterday, so I need to stop here to avoid indigestion.)
Polynovo (PNV)
Matrix Revelations
FY22 result. Record revenue of $41.9m represents a 42% increase from FY21 (BPe $43.0m). Operating expenses of $41.1m were 26% greater than FY21 (BPe $42.1m). Net loss of $1.3m reflects improvement since FY21 (net loss $4.9m). Adjusted NPAT of $0.2m accounts for the $1.4m impairment loss on the sale & leaseback of Port Melbourne facility. Capital expenditure of $0.5m (BPe $1.5m) and net cash outflow of $1.6m (BPe $0.9m). Cash position of $6.1m as at 30 June 2022.
Matrix expansion & strengthening portfolio. BTM sales recorded in new territories within Europe and follow-on sales in key markets (India, Taiwan, South Africa) within the burns & wound care sector. The new MTX product allows application in complex wounds over joints and ulcers (venous leg, diabetic foot). FDA 510(k) clearance is targeted in 1H23 with commercialisation in 2H23. R&D continues within breast & hernia and novel therapeutics including the BetaCell collaboration for treatment of Type 1 Diabetes.
Clinical trials update. Enrolment of 23/120 patients in the pivotal BARDA funded trial in full-thickness burns. SynPath diabetic foot ulcer trial has commenced enrolment in August 2022. Expected completion for both studies is 4Q23. Randomised controlled trial also underway with Flinders University in neuroischaemic diabetic foot wounds.
Investment view: Maintain Buy, Price Target $1.90. We increase our price target to $1.90. The valuation has been generated from a blend of two methodologies: DCF (WACC 10.5%, TGR 3%) and EV/Revenue (8.6x from comps). Key catalysts driving this upgrade include BTM expansion in the Asian market, greater penetration within the US market from existing & new accounts and launch of MTX during 2H23. Product pipeline with SynPath and the novel therapeutics also strengthen the longer term potential for Polynovo.
Recommendation:
Buy
Previous Close:
$1.64
Price Target:
$1.90
Bell Potter Securities Limited
Level 38, 88 Phillip Street,
Sydney NSW 2000
Disc: Held in RL and SM
Good results – and the market reaction so predictable!
$PNV yesterday reported its FY22 results. While overall revenue growth of 42.8% to $41.9m was a touch under consensus ($42.2m, marketscreener.com., n=6 and below my bullish view of $43-$48m), eps and cashflow were slightly negative, where consensus was for both to get into positive territory. None of this concerns me but it explains the market reaction.
In this Straw will cover:
1) the list the highlights from the results
2) details from the discussion on the investor call and
3) my overall take-aways, which includes an assessment of the market reaction which was a 19% drop in the share price!
1) FY22 HIGHLIGHTS
The full highlights of FY22 are (with comparisons to FY21)
- Revenue up 42.8% at $41.9m
- Novosorb BTM sales up 47.6% to $37.6m with USA up 55.1% at $32.1m
- Customer accounts increased by 135 hospitals, excluding distributors
- Key appointments delivered: CEO and Ops Director
- Staff grown from 106 to 152
- US sales team now at 54. There are 9 new starters in last two months and 5 positions remaining unfilled
- R&D team expanded from 1 to 8 (or 9) to intensify new product development, with R&D spend increasing from $3.6m to $5.7m
- 510(k) lodged for MTX with FDA
- BTM registration lodged in Canada. Sales to some surgeons have started and training of staff has commenced.
- Recruitment underway in 3 clinical trials: BARDA Pivotal; US chronic wound insurance reimbursement; and chronic wound study
- $0.62m BTM contributed to clinical trial and $0.52m BTM for humanitarian causes
- Several European markets entered; 3PL deal struck in Belgium
- Property sale and lease back completed
- $3m debt paid down
2) DETAILS OF THE INVESTOR CALL DISCUSSION
Conduct of the Results Call
Those who know Chairman David Williams’ style, appreciate his maverick, straight-talking and unconventional approach. But today’s call bordered on the chaotic. David raced through the slides. The financials weren’t even properly presented. David’s introduction was then followed by a loosely structured conversation with CEO Swami Raote, former Acting CEO Max Johnston, and CFO Jan Gielen all chipping in on various topics.
It was always going to be an interesting call to choreograph but, from my perspective, it needs to be the last one like this. At 1H23 David needs to let Swami take the lead. In fairness to David, a lot of information was conveyed in the discussion which you’ll see below, and we got to see more of the emerging thoughts from Swami that will hopefully set the future direction of the company.
US Sales
The analysts picked up (and prised open in the Q&A) that in the USA, May and June were slower compared with a record April. July was strong again and August is “tracking well”. This signals a slowing of the growth rate in Q4 (and explains why results missed my estimate, which was ahead of consensus.) US growth in H2 was 23% up on H1 (which is 51% annualised), compared with up 55% for the year. While that sounds like splitting hairs, all the rhetoric in previous presentations had conditioned the market to expect accelerating growth in the US. For me, what perhaps made the US number surprising was that H1 was more impacted by COVID-19 access issues than H2, although DW said that the US team had used remote engagement successfully when access was constrained.
Growth in new Accounts (hospitals):
Global new accounts (hospitals) added accelerated. 135 hospitals were added in FY22 compared with 99 hospitals added in FY21. Of these, 80 were added in the US. Sales in accounts existing at the start of the year grew by 88%. This is consistemt with improved access post-COVID and a much-expanded S&M team.
ANZ bounced back after lockdowns impacted H1
ANZ was impacted by lockdowns in H1 (with NZ impacted into H2). With lockdowns lifted, H2 sales were 63% up on H1. The ANZ sales team has now been expanded with a dedicated person in NZ. In 2021 it was over-stretched with one rep covering 30 accounts. Now, the senior reps are supported by associates, a model which is working well and creating entry-level opportunities.
ROW – what’s going on?
It’s a tale of two very difference parts. ROW consists of UK&I and what is now quite a long list of continental European countries. Overall, sales were up 50% from $1.6m to $2.4m, as follows:
- In UK&I the direct sales model is getting traction. UK and Ireland achieved sales of $1.3m, up 185% y-o-y from $0.5m. 31 new accounts added from a salesforce expended to 9 from 5.
- The Rest of “ROW” (Continental Europe) was flat: Backing this out, all these other countries achieved $1.1m, essentially flat from FY21, but apparently it “improved in 2H22”. (I asked a question why the distributor model in Europe is clearly not getting traction, but this question was not selected in the Q&A! :-(
Capex down to $0.5m from $3.6m
With the second production line built and undergoing validation, capex has fallen significantly. It was emphasised that this is a capital-light business, and they are keeping spare production capacity in place to underwrite future growth. FY23 capex will also be low.
BARDA
BARDA – the US government agency – is funding a $15m multi-year trial to generate the data that, if successful, will lead to $PNV being stockpiled by the US Government, so that there are strategic reserves in case of a major disaster. Swami has experience from J&J in working with BARDA and he said that the focus and attention BARDA are giving to BTM is as high as he has ever seen (including on vaccines!) Down the track, this offers the potential for some material boosts to sales. BARDA is working with $PNV and the FDA to get approval for BTM for second-degree burns. This would give a more general market-wide boost to BTM sales.
Product Development
Good progress on all 3 “pillars”
- Advanced Wound Care: Focussed on extending the current product beyond burns. The idea is to improve connections with innovative surgeons who are surging ahead. R&D will work to tailor the product to their needs (e.g., smaller sizes; useful shapes).
- Implantables: 4 prototypes have been developed for hermia; and one “breast sling”. No specifics provided on timing for launch.
- Therapeutics: there is “enormous interest” by external partners (pharmaceuticals) to use drug-impregnated BTM as a local drug delivery vehicle. David and Swami recalled a recent trial case study from Adelaide in treatment of a Type I diabetes patient, which was transformational for the patient. David in his latest red jacket, provided his characteristic simulation of the procedure. (My note: If this gets traction, the opportunity is huge, but we need to think of timelines in terms of 7-10-+ years, but it does present very material, long-term blue-sky).
The focus in the short to medium term will very much be on Advanced Wound Care, because the regulatory hurdles are lower, time and cost to market is lower, and the synergy with the existing sales and marketing capability is very high. Several clinical studies are being funded to get the data to support a broader set of wound indications. The sales reps need this data to influence their customers.
Finances
There is $6m cash on hand, down only $1.1m from YE21. Last year $PNV said “The business will continue to reinvest cashflows to expand market share in existing markets, enter new markets, and develop new products.” That is exactly what they have done. In addition, $3m of debt has been paid down using proceeds from the property sale and lease back.
I think this is where the market might have been expecting more. Looking back at FY21 results (slides 28 and 29), the net profit and cash flow trend charts set the expectation (reflected in consensus forecasts) that both cash flow and net profit would be clearly in positive territory. Neither were.
That said, H2 was operating cash flow positive compared with H1 s;ightly negative.
In Q&A, when asked to comment on the sufficiency of capital, DW was more circumspect than this time last year, deferring to Swami. That was a good call because he has to leave space for Swami to put forward his growth strategy, To close the option of access to capital would have been a BIG MISTAKE. IF Swami is smart (and I think he is) he would have agreed this with the Board as a condition of joining. Of course, with debt paid down and rapidly growing revenues, $PNV has greater debt capacity in FY23.
Jan reported that margins were continuing to increase, due to price increases to offset rising costs and higher facility throughput. He reported gross margins are up 0.2% to around 95%. There was a brief discussion that the market can bear higher prices if necessary, because the competing products are more expensive and their treatment and post-treatment support is more complex and therefore more costly. The healthcare economics equation is about more than just the product cost.
Sales and Marketing – further insights
The imminent Canadian launch will adopt a hybrid sales and marketing model. A distributor partner has been selected with 17 identified, experienced sales agents. Ed, the SVP S&M Americas will retain responsibility for the sales in Canada, including training and supporting the outsource partner. David, Swami and Max were all very positive about the prospects for this model, and they expect rapid traction in FY23 as a result. Swami said that some surgeons in Canada who have already carried out some procedures have reported "tremendous results". Surgeons are already placing orders “at their own risk” ahead of product registration.
Generally, we heard repeatedly that sales and marketing for BTM requires a “high support” model. Swami said that many distributor partners wouldn’t work, because they see their job as “getting to the hospital purchasing desk” to take orders. With BTM, the relationship is between the sales agent and the surgeons and nurses. (I think this explains why Europe is treading water at the moment. They know what to do, they just didn’t want to talk about Europe because they don’t have a solution there yet!)
In the USA, Max mentioned that 2 GPO deals are in the pipeline, with one big one close to landing. However, Max and Swami reinforced that GPO deals must be underpinned by the Sales and Marketing people on the ground at the hospitals for the reason discussed above.
On sales and marketing, we heard a repeat of prior messages: that new sales agents don’t do that much in the first 6 months, but that by 12 months they are contributing more than their costs. This time, however, the messages were somewhat more nuanced pointing out that it depends on a range of other factors. These include how many existing accounts are in the territory and how mature they are. Going forward, it will be important for both management and analysts to learn how to model sales growth expectations from S&M investment.
We heard some more of Swami’s ideas
- He believes that BTM is a superior product to biologics: better clinical outcome, easier to use, better economics (for the hospital), better patient experience in treatment. He cited that staff who have come across from Integra and Recell support this.
- He wants to leverage a November Conference in India to stand up a team there and launch. “Eventually, there will be more procedures in India, however US will remain biggest for revenue, due to much lower revenue per procedure.” He doesn’t want to wait to strategize on this, but he wants to get going. DW said it had been discussed at the Board meeting that morning. Swami said that hospital-sourced infections is a big problem in India, so BTM has big advantages because of the reduced risk of infection in its use.
- More generally, Swami is focused on how $PNV can accelerate global adoption, add new markets, broaden from burns into trauma (DFU, oncology, etc.), and move beyond the hospital.
- He believes in the US sales team can “take the trajectory up”.
- He is scanning for acquisitions that can enhance market access. They are not looking for products, but access to surgeons, particularly podiatric surgeons in the US (who usually operate out of stand-alone facilities, not hospitals, unlike in Australia). (Incidentally, DW asked investors to stop writing to him to suggest they buy $AVH or $ARX. They don’t want biologics in the portfolio.)
- He said that if they had a blank sheet of paper his market priorities would be USA, Japan, China, Western Europe. So, I think we should see some changes over the next year. When challenged in Q&A over whether the priority should be to bed down existing markets before expanding, Swami’s view was that this wasn’t necessarily valid, as what goes on in one market doesn’t detract from another. (I believe that Swami will bring to $PNV an impressive contacts book of sales and marketing managers from his international experience, and that this might prove to be a huge asset to $PNV.)
- In terms of product development, he wants $PNV to get even closer to the surgeons and get their feedback on how they want to use the product. He is clearly an advocate of customer-driven innovation. An example of this is regional Australia. He asked, how can procedures and product be evolved to allow the patients to come in to hospital for one treatment, and then not have to return to hospital? Swami cited what he called “unicorn surgeons who have already taken BTM to more places that we have”.
- He noted that applications involving implants will have a longer regulatory hurdle to overcome (lots of experience from J&J!), and he believes that engagement with regulators is very important.
3. MY OVERALL TAKEAWAYS
On the Results
By any measure, these were good results in USA, ANZ and UK&I; even if it is unclear why May and June experienced softer growth in USA. It is not a concern at this stage, because on such a fine timescale you’d reasonably expect some variability month to month.
Europe (distributor markets) requires attention, as the model is not capturing the opportunity. They know it. They just don’t have a plan yet.
They did what they said they would do: using cash generated to grow sales and marketing and expand product development. This is exactly what they should do. In addition, some debt was paid down, creating balance sheet capacity.
The Future
Swami is passionate about the product and the market opportunity, and he wants to move quickly. Given what $PNV has proven to date, it makes sense to go much harder on key markets in Europe and Japan and (I defer to Swami’s view about ...) China.
This is going to take a big investment in building out the global sales and marketing capability. For example, it has already taken three years building in the USA, and they have further to go. It won't be so easy elsewhere, but fortunately, Swami have very relevant experience outside of USA and Europe - a huge asset to the company. It will likely involve a mix of direct selling and deals with appropriate contract sales and marketing entities/distributors. It is unclear to me what the cost of this will be, but $PNV should be willing to raise debt and capital to fund it. Growth at these margins will drive shareholder value.
I expect Swami will go through his own adjustment period. $PNV doesn’t have a fraction of the depth and breadth of capability that Swami was used to in J&J. So, he will have to lead in a very different environment. It is important that David supports him, but also doesn’t stifle him. This is a key potential risk to key an eye on, but David is well-aligned with investors.
Finally, the Market Reaction
In short, the 19% SP drop is entirely unsurprising for the reasons I foreshadowed in my Straw the evening before results.
Since the Q3 trading update, which received a muted SP response, the SP had rallied 77% from $1.13 to close at $2.00 prior to results, standing 22% higher than market consensus. This was driven by DW’s share purchases, buying by short sellers, and a positive response to Swami’s appointment.
The results in terms of revenue, net profit and cash flow were a slight miss on consensus, so it is entirely unsurprising that the price has dropped right back to the consensus level. The market has behaved precisely as you’d expect.
Furthermore, not only is $PNV one of the more shorted stocks on the ASX, its historic volatility makes it a traders favourite. Don't invest in $PNV if you don't have a strong stomch for volatility. We should expect this to continue until performance becomes more predictable. Here, I think David and Swami have a key role to play in improving the quality of investor communications. This subject is worthy of a separate note.
All that said, I believe Mr Market has handed investors another opportunity to get onboard at what I believe is a large discount to fair value.
CONCLUSION
With $PNV now 6% of my portfolio and my high conviction unchanged, I am a HOLD for now.
However, should there be any further SP weakness below $1.60, then I will increase my holding.
I believe the market is significantly under-valuing $PNV. However, thee is real valuation risk in sales and marketing execution. In the appointment of Swami Raote I believe the risk has reduced, and I will be eager to observe how he settles into the role over the next 12 months.
I’m looking forward to the AGM on 28th October and to FY23 – which will hopefully be a “cleaner” year without all the caveats and stories about COVID-19 obscuring the underlying performance of the business.
(My valuation to following in a week or two.)
Disc: Held on SM and IRL
I am very much looking forward to hearing Swami and David deliver the $PNV FY22 Results tomorrow.
Whatever the results, it will be hard to call the price action. Shorts (graph below) have been reducing given positive updates(Q3), DW share buying spree and a strong CEO appointment. However, taking a 3-year view they remain high at 6.78% as of last week - there's a possibility for strong movement either way, depending on response to results.
Analysts don't know what to think or haven't updated. SP is 22% above consensus (n=6, marketscreener.com, range $1.11-1.90). I'm at $2.80 (central case, DCF, will update on SM after I have digested the results).
Consensus on sales is $42m, but my estimate is $43-48m range.
With DW's last purchase reported on 9 June, and with his strong buying through Q4, I assume the sales trajectory will be strong.
Cash flow will be at or close to breakeven, before including proceeds of real estate sale.
A few uncertainties impacting cash flow are impact of wage increases and inventory changes to support sales, although according to Swami $PNV is being sought out in the sector as employer of choice and according to David, supply is easy due to very high %GM, so no need to hold significant local regional stocks now that borders are free flowing again.
Here is the link to the results call, tomorrow at 2:00pm:
https://services.choruscall.com/mediaframe/webcast.html?webcastid=DVUbmPqJ
Disc: Held.
On both SM (18%) and IRL(7.4%), $PNV has become my largest position, even after trimming along the way. My conviction on this firm is such that I am going to let my winners run.
from www.shortman.com.au

First patient enrolled in daibetic foot ulcer Randomised Controlled Trial.
Enrolment targeted to be completed by Apr 2023, and trial endpoint measurement is 12 weeks. So all the data for the primary efficacy endpoint would be in by July/August 2023. I think they last said targeting approval by end 2023, but I wouldn't fix on that. A lot depends on i) how long enrolment actually takes (Apr-2023 might be conservative?), ii) how strong the efficacy result and iii) resourcing and priorities at the FDA.
Overall, good to see this next major indication for the platform making progress.
Disc: Held on SM and IRL
https://newswire.iguana2.com/af5f4d73c1a54a33/pnv.asx/3A598154/PNV_CEO_Appointment_Swami_Raote
$PNV have just annouced the appointment of Swami Raote as the new CEO, following a search process that David has been running for 9 months. As reported, the new CEO appears to have a track record of both operating at greater scale and driving growth, and is well-credentialed in healthcare including medical devices in a world class company (J&J).
Market has responded positively so far today with SP up 6% at time of writing.
David is going organise a meeting in the next week or so to introduce Raote to shareholders.
Disc: Held on SM and IRL
Sale of the Port Melbourne HQ is completed. The $6.35m in the bank will see of any chance of a further raising for the current operation. This was well communicated and expected, but good to see it delivered and we can now look forward to $PNV becoming free cash flow positive.
Disc: Held on SM and IRL
DW at it again. Is he trying to squeeze shorts, keep it in the ASX200 or is he reading strong sales reports?
More director buying. David is loading up some more.
In this straw I wanted to provide some colour around Nnyck777’s very informative straw on ##polynovo FY21 results by sharing my observations from the recent investor call. The points I make underpin the assumptions in my valuation, which I detail in this note.
Over recent years, there has been commentary from time to time on how expensive the PNV SP is. However, I believe that the short-term horizon of most analysts is under-valuing the opportunity. My analysis yields a range of values from $2.75 to $4.50, leading to a central case valuation of $3.62.
The FY21 Presentation
To begin with, Chairman David Williams announced a “red jacket” day to celebrate that PNV “is now profitable”. Strictly, this is an adjusted profit of $0.26m, excluding certain non-cash items (share based payments, D&A and unrealised FX changes), or a statutory loss of $4.6m.
For a company so early in its growth runway, this was hardly the message to lead on. However, it underscores a message David and CEO Paul Brennan repeated throughout the presentation in that 1) they are demonstrating capital discipline (no capital raises in prospect) which is enabled by 2) the product is growing quickly and has a very high margin (>90% margin on manufacturing cost) and 3) facilities both for BTM Novosorb and the yet-to-approved hernia product have been built and commissioned, and can support production for the next few years. So essentially, sales will fund both the build-out of the global sales force and R&D of a platform of potential follow-on products and applications described by Nnyck777, with the near-term focus on the hernia product.
The core of my thesis is that PNV has established a technology platform that will deliver a range of medical device products which are already identified, so the future spend is essentially development rather than research – a lower risk proposition. BTM is generating a body of clinical evidence about its effectiveness in journal papers and it is instructive to watch some of the webinars being given by opinion leader surgeons using the product, indicating that it is likely to become the new standard of care.
For me, the big message for FY21 was that PNV achieved sales growth of 32% in a year when their major market in the USA and recently-launched markets in UK and Europe had very limited opportunities to get in front of surgeon customers as a result of COVID-19 lockdowns and the impact on hospital access and priorities - a major drag on new accounts.
In the Q&A to the presentation, the analysts were trying to get a handle on sales growth for FY22. CFO Jan confirmed that headcount would grow from 106 to around 140, which would indicate a sales and marketing team growing from 52 to 70-80. Management was cagey about the revenue expected, however, David explained that they expect each salesperson to do $0.75m to $1.00m sales (once up and running). Jan (CFO) made it clear that sales growth would not be 100%, but it would be significantly more than the 32% in FY21, citing access to hospitals having already moved from 50% in FY21 to 70-80% of normal at the start of FY22. Jan also referred to the July release, which reported June at 3.3M BTM sales, and US sales of $4.9m in 4Q21 over $3.3m 4Q20 – a c.50% increase. Taking these elements together: re-opening, new jurisdictions, new territories, new accounts, increased usage in existing accounts, new indications supported by a growing salesforce, salesforce experience supported by a growing body of literature leads me to estimate revenue growth of 50% and 75% anchoring my bear and bull cases for FY22.
Large Addressable Market
In previous presentations, PNV have touted their markets as:
· $1.5 bn dermal scaffolds (CAGR 10%)
· $3bn hernia devices (CAGR 9%)
· $3bn Breast Augmentation/reconstruction (CAGR 9%)
· Undefined – drug elution/ chronic disease management (Note that the overall controlled release drug delivery market is of the order or magnitude of $40bn, albeit with many products in the market)
So, ignoring the potentially very material drug elution market, PNV is developing a platform of products serving a market which is currently c $7.5bn and likely growing to c.$20 bn in 10 years.
In my valuation scenarios, I assume sales grow to $875m (low) and $1,500m (high) by 2031, which would be a market share of the order of 4.0% to 7.5% across the dermal repair, hernia device and augmentation/reconstruction markets, with any drug elution applications an upside. While they are likely to achieve a greater share in dermal scaffolds, I am happy with the conservative assumptions which allow for emergence of competition, margin pressure over time as well as failures to achieve new indications.
In modelling the sales growth, I start at the low (50%) and high (75%) cases in 2022, and trend down to 20% in 2031, with around 4-5% implied in the CV period of the DCF model.
Manufacturing and Supply Chain
BTM has a very high (>90%) margin on its cost of manufacture. I assume 7% cost of sales excluding personnel to cover manufacturing, inventory growth and logistics costs, which probably equate to 12-14% once personnel costs are included. (Remember, this is a very high value product with a 10cm x 10cm square selling for $800-900!)
Sales and Marketing
With around 50% of workforce currently in sales and marketing, David declared that each head achieves $0.75m – $1.0m in sales. Going forward, sales force coverage of territories, sales force effectiveness and the performance of distribution agreements will be key. For modelling purposes, I assume a 100% direct sales model (on the basis that if distribution agreements under-perform they will be replaced). Over time, salesforce effectiveness will increase as i) more surgeons become repeat customers, ii) the number of applications increases and iii) as the product portfolio expands (i.e. more sales opportunities per unit visited). In my model, I assume the sales force scales at a factor of 0.8xsales. The means that sales per head in $2022 goes from $0.62m-$0.73m in 2022 to $1.20m-$1.47m in 2031 (in $2022). This is one of the key assumptions in the valuation.
R&D and Investment
Although having signalled a reduction in capex in FY2022, over time, manufacturing facilities will have to scale with growth and with new products. I assume capex/sales is sustained at 5%. Equally, PNV will need to continue to invest in innovation, and I assume R&D at 10% of sales, which is in line with other high innovation firms like CSL.
By continuing to invest in R&D, PNV will have a platform of products which will continue to unfold for decades to come. Accordingly, I have put a FCF growth rate of 6% in the continuing value period (2032+), which I consider to be conservative for an innovation-led company. (Again, this is why I hope David Williams doesn’t bang on too much about his “red jacket day”. He can bring out the red jacket again, when they get $200m in sales!)
Pricing
I assume pricing remains in line with currently levels, across products. BTM already has a number of competitors and yet is achieving good levels of adoption. My market share assumptions for 2031 are on the low side and therefore there is an opportunity for a low pricing/higher share scenario depending on how the market unfolds. In any event, pricing is well below the main competitor Integra (reported elsewhere on Strawman).
Bottom Line - Valuation
At end 2020, the market got a little over-enthusiastic about PNV with SP getting to $4.00. ( I exited at the time.) However, having updated my valuation based on the lastest report, I have arrived at a target SP of $3.62, with a range of scenarios from $2.75 to $4.50. The wide range reflects the real uncertainty for a company very early in its growth runway. My valuation yields a P/E of 50 in 2026 (not much higher than CSL which is 10-15 years ahead!) with earnings growth 80-100% at that time.
Key Risks
With the Delta wave rolling out around the world, reimposition of restrictions would slow down sales growth and act as a drag on reinstating full access to existing and potential customers. Dermal repair procedures are often (mostly?) not elective, however, if sales reps are restricted in gaining access, this will be a drag on new accounts. I’ve modelled this as one year of 35% growth, which moves the SP range to $2.15 - $3.50.
Other risks include failure to gain approval for hernia and other applications, which as the discussion above indicates would create a company focused on dermal repair, with a correspondingly more-focused cost structure. I think the low end of my SP range covers this.
Comparison with brokers
According to marketcreener.com, 7 brokers have an average target price of $2.35, with a range from $1.80 to $2.80, so my range of $2.75 to $4.50 is definitely bullish. Again, I think the skew may arise from the shorter term horizon in the broker methodologies.
[DISC: Held in SM and IRL. I will be buying this week IRL to increase my position given the current SP weakness.]
Edit: changing range $2.50 to $4.25 in section "Bottom Line - Valuation" to correct value in second para. which is $2.75 to $4.50, which are the correct values from model. Soz.
Post a valuation or endorse another member's valuation.


