Consensus community valuation
Straws are discrete research notes that relate to a particular aspect of the company. Grouped under #hashtags, they are ranked by votes.
A good Straw offers a clear and concise perspective on the company and its prospects.
Please visit the forums tab for general discussion.
It seems the market prefers knowhow over fanfare!
Resignation of Chair
PolyNovo Limited (ASX: PNV) (PolyNovo or Company) today announces that its Chairman, Mr David Williams, has resigned from the Board with immediate effect.
Mr Williams will not stand for re-election at the upcoming Annual General Meeting and the resolution for his re-election is withdrawn.
The Board has appointed current Non-Executive Director Mr Leon Hoare as Chair. Leon brings very strongly aligned industry knowledge acquired over 30+ years in executive roles and extensive leadership experience in wound management and related medical technology sectors. Leon also has extensive PolyNovo board experience, which in turn will support the new CEO, who commences in December. Leon’s appointment is timely as he will be stepping down from his current executive role at the end of December.
This development, together with the appointment of Rob Douglas to the PolyNovo Board, follows PolyNovo’s previously announced board succession plans and governance review, in line with the Company’s ongoing commitment to board renewal.
PolyNovo’s Board acknowledges Mr Williams' significant contributions and leadership during his tenure. Chair, Mr Leon Hoare commented: "On behalf of the Board and shareholders, I would like to thank David for his 11 years of dedicated service as Chair. Under David’s leadership, PolyNovo evolved from an early-stage medical device venture to a global medical technology company. The Company achieved record growth in sales and expanded its presence to 46 countries, delivering innovative wound care solutions to patients worldwide.”
Mr David Williams said: "It has been a privilege to serve as Chair of PolyNovo. I am extremely proud of what we have accomplished, especially the number of lives we have changed and saved. I am very pleased with PolyNovo’s new CEO; Bruce Peatey, new director Rob Douglas, and new Chair Leon Hoare.
There’s a bit of excitement in the market today on the back of PNV’s Q1 FY26 Trading Results announced yesterday. Evan’s & Partners have now rated PNV as a Speculative Buy with a $2.65 price target. Macquarie retained its Outperform rating and the $2.00 price target. Overall, I’m quite happy to hold the business. If revenue continues to grow at this rate it might look cheap in a year from now! I am expecting earnings growth and ROE to be both around 25% going forward. It currently holds more cash than it has debt, so it’s starting look like a reasonable investment again!
Trading Results Q1 FY26 (unaudited)
PolyNovo Limited is pleased to provide unaudited trading results for Q1 FY26.
- Q1 FY26 Group sales were A$34.7m up 33.3% on STLY of A$26.0m
- U.S. sales were A$27.4m up 33.4% on STLY of A$20.5m
- Rest of World sales were A$7.3m, up 33.1% on STLY of A$5.5m.
- NovoSorb MTX sales of A$2.9m up 174.8% on STLY A$1.1m
- Strong collections from U.S. customers, with days sales outstanding (DSO) averaging 61 in Q1FY26 down from 95 DSO in March 2025.
- The new R&D Innovation Centre is complete and operational, and capex is finished.
- Building works of the new manufacturing facility are now complete with circa A$2.5m in capital expenditure required to make operational.
- Q1 FY26 was EBITDA positive and cash on hand on 30 September 2025 was A$23.2m.
Chairman, David Williams said: “There is a lot to like and be proud of in the continued growth in sales, hospitals supplied, patients treated, countries supplied and surgeon engagement. Beyond these indications of continued growth, the real excitement for me is in:
• The near-term possibility of moving into the U.S. outpatient market and supporting plastic surgeons, podiatrists and home care. Proposed changes to U.S. pricing in CY26 will reinforce our market opportunity. Our new CEO and Board Director will add strength to that push.
• NovoSorb MTX has had tremendous growth off a low base, but our team are very bullish on the prospects in the U.S. and the rest of the world.
• I have observed before the slow start to sales in places like the United Kingdom but then followed by a ramp in sales. It is very exciting to see in Q1 FY26 that ramp in countries like Turkey up 97%, Germany 269% and Canada 63%
Held IRL & SM
You would think David would be able to find a more flattering picture.
Maybe not.
Today I've undertaken a quick deep-dive (if there is such a thing) into Friday's announcement from $PNV on the application of Novosorb BTM in the treatment of diabetic food wounds (DFW).
This is a readout of a quick, initial scan to understand how excited I should be about findings.
TLDR: there is something here to consider, but I'm not in a rush to act.
I've investigated the following questions.
- How significant is the clinical result?
- How large is the market opportunity (starting with the US)?
- What other treatments are already serving this market?
- How might $PNV access the opportunity in the US?
- What's my overall view / implications for value?
1. How significant is the clinical result?
The acceleration in the time for the wound to heal is very significant: 191 days for BTM vs. 319 days for the SoC.
The ultimate improvement in outcomes is not statistically significant: 12 month healing rate of 66.7%for BTM+SoC vs. 56.5% for SoC but only with a p=0.48. Which means the results are barely distinguishable. i.e., a failed endpoint, were this a registration trial.
There was no significant difference observed in 12-month amputation rates.
So this looks promising, but it is important to understand that while the SoC is negative pressure wound therapy (NPWT) only, clinical practice in the US is already using a large number of alternative dermal substitutes with NPWT. So while the trial looks promising against the SoC, the industry has already largely moved beyond the SoC.
And here's the problem: I can find little if any evidence of clinical trials for these wounds pitting SoC alone against SoC + ny other dermal substitutes.
If fact, the whole field of how the various treatments have achieved their registrations and reimbursement codes is somewhat of a mystery to me and require further investigation. But that's for another day.
2. How large is the market opportunity (starting with the US)?
The overall DFW market is huge, and the segment of interest being post-surgical diabetes related neuropathic/neuroischimc wounds is anywhere from US$1.0 - US$2,5bn per year.
Importantly, the proportion of this market attributable to dermal substitutes appears to be in the order of US$0.3bn to $1.0bn p.a. (Sorry for the wide range, but the calculation combines varied factors, ranges and estimates)
3. What treatments are already serving this market?
In short, a lot. And more than I was expecting when I started the research.
I have (with support from my trusty BA) identifed no fewer than 23 existing dermal substitutes with relevant reimbursement codes, using a wide range of technologies including Novosorb BTM.
What? Novosorb BTM is already on the list? The answer is yes. And the reason is that many of the products appear to be being used in DFW using a more general registration code, and not exclusively a DFW code (or codes, as there are several procedures). That explains why Novosorb BTM is already being used, as it is being used under its more general registration for complex wounds.
How can this be, Well, some of the Novosorb super-users are general surgeons or trauma specialists, and they do sometimes treat DFW. So it is natural that they would give it a go. This is also consistent with the legion stories we've heard from David and recently departed Swami about "surgeon-led innovation".
The point is, it currently depends on surgeon initiative. $PNV reps. can't go in and say "Use this", "here's the evidence", "here's the reimbursement code", "order here". The trial is a step to changing that.
4. How might $PNV access the opportunity in the US?
It seems that much or even most of the DFW treatment is performed in the US by specialist Podiatric Surgeons. (DW has spoken about this repeatedly over the years).
The bad news for $PNV is that I think the burns, trauma and general surgeons in the locations which are BTM super-users perform a relatively small proportion of DFW treatment. Indeed, a large amount of the care is in outpatient settings. (Again DW has spoken about this before.)
I imagine (I don't know) that this means there will be only a limited overlap with the existing relationships and accounts for the salesforce. Resources will have to be reallocated or added, and new relationships built.
But importantly, many of these HCPs will have their existing preferred treatment methods, and will rely both on clinical evidence and economics to switch.
Now DW's most recent messaging on the costs of treatment starts to make sense. BTM is a relatively cheap product compared with many dermal substitutes, and has extremely high gross margins.
Therefore, I think if they can get the clinical evidence lined up, they could mount an assault on this market. But it will take condierable effort and time.
5. What's my overall view / implications for value?
I don't think attacking the DFW market in the US is going to be a quick process for $PNV.
By the looks of it, while the time-to-heal measure is good comapred with SoC, we don't know how it compares with other dermal substitutes. So it is not clear to me how $PNV can persuade a rusted-on podiatric surgeon to switch from their current practice.
The sales force will have to be augmented or retooled to go after this segment. That won't be quick.
The space already appears to be crowded with a vast array of choices for surgeons.
If you pushed me, I'd say that over 5-7 years, they might claw their way to a 10% market share in this segment, which could be anywhere from $US30 to US$100m incremental sales, as a BULL CASE.
That compares with FY25 US BTM sales of around US$57m sales, so it is a material opportunity and it has the potential to extend the growth runway in the US market.
Final Comments
These are very rough, back of fag packet calculations. For example, if existing practices are locked in, accessing practitioners is difficult, and the clinical data is judged not to be compelling or significantly differentiated to what the surgeons think they are already achieving, then maybe $PNV fights to get 5% or goes harder on price to get a bigger share. In that case it might not amount to very much ... maybe $US15m to US$50m p.a. incremental revenue in 5-7 years.
Alternatively, if the clinical data is built on over time and is strong, and it plays into a weak comparison set, and if US opinion leaders embrace the treatment, and if $PNV executes an effective sales strategy (perhaps partnering with a leading distributor in the podiatric market), them maybe they can get a bigger share, faster, pushing closer to US$100m p.a. in 5-7 years.
But there are a lot of "ifs" to get to that number.
It is impossible for me to be more definitive from this quick look. But I think I have sketched some bookends to think about. For sure, this looks interesting and seems to be an opportunity that DW and his team will be applying themselves to.
But I think there are a lot of both clincal and execution questions, so based on what I've learned, I'm not getting overly excited about it, just yet. It is clear that this is a competitive market with many treatment alternatives. Many more than I realised at the start of today.
Keen on the views of others who follow this sector.
Disc: Not held (yet)
DW third day in a row buying shares on market. Is this history repeating itself? With over 12% of PNV held short and PNV dropping out of the asx 200 could another short squeeze be on the cards?
Very tempted to buy as this may run quite quickly. A few good news announcements would help.
David Williams and Leon Hoare have both bought shares in the last few days, with Mr Williams getting a another quick buy in this morning before a "pause in trading"
Trading in the securities of the entity will be temporarily paused
pending a further announcement.
Trading in the securities of Polynovo Limited (‘PNV’) will be halted at the request of PNV, pending the release of an announcement by PNV.
Unless ASX decides otherwise, the securities will remain in trading halt until the earlier of:
• the commencement of normal trading on Wednesday, 3 September 2025; or
• the release of the announcement to the market.
The halt in trading was requested by PNV to address press speculation, as written in The Australian on 31 August 2025 article titled “Aussie medtech’s unlikely boost from RFK health crackdown, of a positive change in U.S. reimbursement of outpatient wound care.
I asked ChatGPT for some insight into the RFK health crackdown and what it means for Polynovo. Here is the summary:
Summary: RFK Crackdown → Upside for PolyNovo?
- RFK Jr.’s crackdown: Aggressive regulatory and budget reforms in healthcare and food industries—reformulating reimbursement models and tightening FDA oversight.
- PolyNovo’s advantage: A lean, lower-cost product that may become more competitive under CMS’s flat-fee scheme, increasing its attractiveness to U.S. outpatient clinics.
- Current footnote: PolyNovo has paused trading pending a company response to media coverage.
How PolyNovo Fits In (and Potential Benefits)
- Medtech Opportunity: The U.S. Centers for Medicare & Medicaid Services (CMS) is proposing a shift in reimbursement for outpatient wound care—from percentage-based pricing to a flat fee of USD 806 per square inch starting January. That’s a move meant to cap expenses currently reaching up to USD 2000 per square inch.
PolyNovo’s Position:
- Its NovoSorb artificial skin costs are understood to be well below USD 806, even factoring in a possible 10% tariff on Australian-made products.
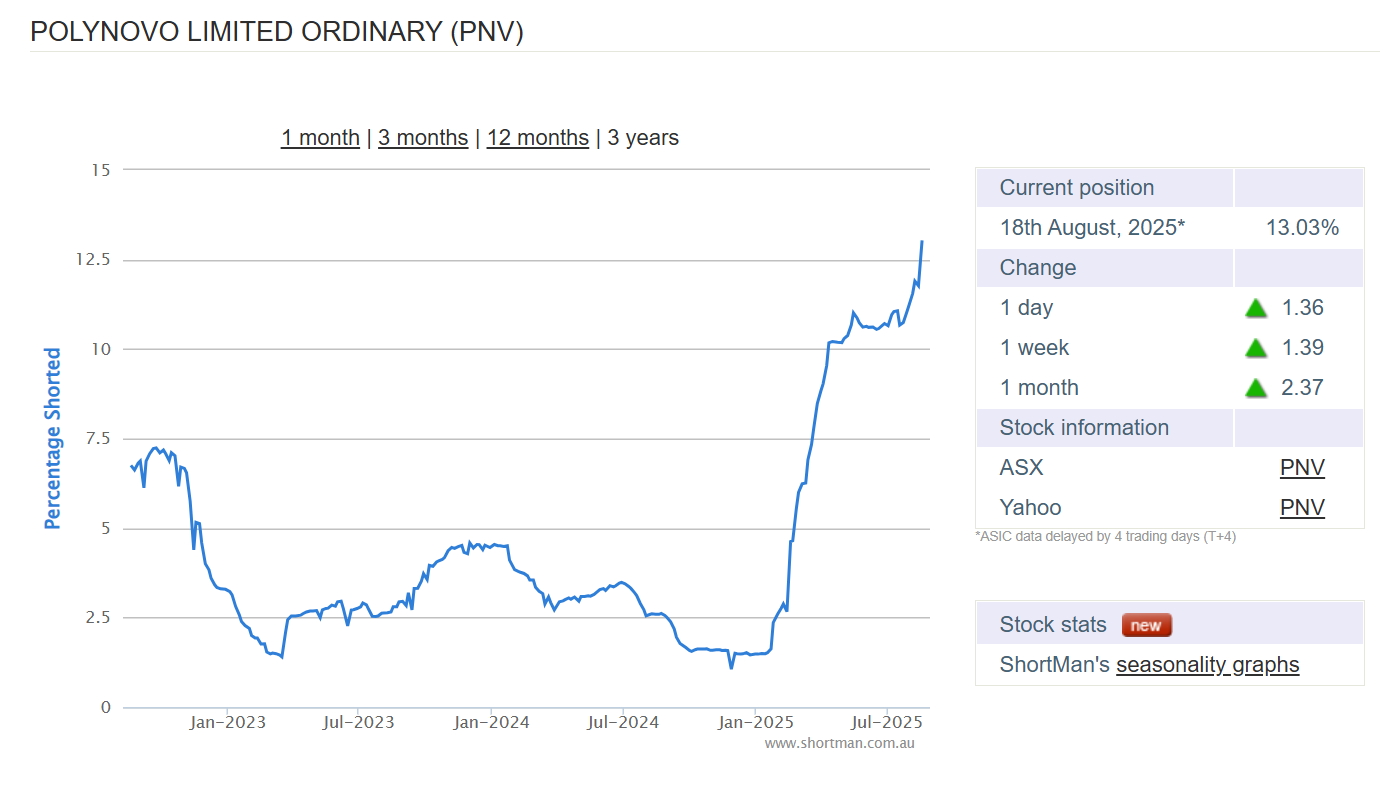
With the FY25 results for $PNV due this afternoon, it is interesting to note the short position (below).
3-year high - while the SP languished at $1.10.
Could be interesting.
Disc: Not held
July 2025
$1.75 ($1.27 - $2.20)
A further update to my $PNV valuation, following the trading statement released after the close today.
I've rerun my model, by just putting in a series of more modest revenue growth rates (which appear to have stabilised somewhat H2 over H1).
NOTE: this is not a complete re-rerun of the valuation, but it is a better estimate than my January edition, which was too optimistic on revenu growth and which I therefore want to "correct".
BIG HEALTH WARNING: I need to look at the FY financials when these are available in a few weeks.
That said, DW's forecast for EBITDA of $11.2 - $12.4m is eerily in line with my central case ($11.7m) - but remember, even a broken clock is exactly right twice every day! The cash part of my model for FY25 isn't so good, so something ain't quite right.
 Disc: Not held - staying on the sidelines until I can see the full picture.
Disc: Not held - staying on the sidelines until I can see the full picture.
January 2025
$2.20 ($2.00-$2.50)
This is a placeholder following 1H FY25 revenue report, which appears to take higher value scenarios off the table.
This is a rough estimate because cost structure can make all the difference so will update after the full financials are in.
August 2024
$2.60 Range = ($2.30 - $3.50)
Full model rebuild following FY24 results. Results of valuation broadly in-line with previous modelling - just a simpler model, which will be easier to update in future.
In FY24 $PNV passed through the profitability inflection - so you can still get pretty much any valuation you want, depending on what you believe.
Lot's of upside not captured in this range, but the downside is better characterised.
See today's straw "Valuation" for further details, assumptions and outputs (27/8/24).
----------------------------
February 2024
Manual adjustment by +5% to reflect growing evidence of capital discipline, as revenue growth strongly outstripping cost growth.
(January's improvement accounted for revenue numbers).
Full update after FY results in August.
----------------------------
January 2024
Raised from $2.00 to $2.12
Quick valuation update following 1H FY24 Trading Update.
Overall, tracking in line with my expected valuation.
-------------------------------
September 2023
Value $2.00 Range [$1.63 - $3.30]
See Valuation Straw for details
-------------------------------
September 2022
Value $2.46 (Bull $3.28, Bear $1.63)
This updated valuation replaces my earlier valuation from a year ago of $3.62 ($2.50 - $4.25). I make a comparison of the two valuations at the end of this note.
Detailed 10-year DCF developed to understand sensitivities and key value drivers.
MAJOR HEALTH WARNING – you can get pretty much any result you want in DCF modelling. Therefore, in each year key metrics of sales growth, and key metrics (employee expense, R&D, Overhead, investment) and resulting EBITDA, EV, p/e and EV/EBITDA have been extracted and are tabulated below.

The estimate is driven by the following key assumptions, which are constant across BULL and BEAR scenarios.
Key Assumptions - Common to Both Scenarios
1. Sales and Marketing
Modelled sales are driven by growth of the global sales and marketing (S&M) organisation starting in USA, ANZ, UK&I and Canada (“Initial direct markets” or IDMs)
Sales per S&M Employee reach an “Experienced Average” after two years, with ramp up to “Experienced Average” of: 0% in 0-6 months; 25% in 6-12m and 75% over 12-24m. This has been modelled by data on headcount and sales over the last three years and extrapolating various statement from DW and management on investor calls over the last 2 years. The model will continue to be calibrated over time. (Note: Aroa CEO has said that the ramp-up takes 3 years. This is a sensitivity to be tested.)
The current average sales per “Experienced Average” S&M employee is $1.0m p.a. (Note: This doesn’t history match to the data for 2020, 2021 and 2022 because of access issues during COVID19. Results in FY23 will be the first test in a "clean year".)
S&M build-out extends to Europe, China, JKT, India, Middle East in FY23/24/25
Ultimate growth of sales teams by 2032 in IDM markets is tested by an assessment of number of large hospitals and burn units in each market, to ensure number of accounts per territory at maturity does not fall below 6. i.e., the model explicity tests that it does not over-saturate the market with S&M staff.
As indications extend from burns to wound care and onwards to reconstruction, the amount of work per account will increase.
Sales and marketing footprint in 2032 has 53% of sales force in IDMs, 24% in continental Europe and only 14% in JKT and China. Clearly, there is scope for significant upside, particularly towards the end of the decade.
By 2032 the global S&M organisation comprises c. 500FTE, about 55% of the total workforce, which is not very different from the mix today.
2. R&D expense grows y-o-y. However, as a % of revenue, non-employee R&D expense falls as a % of rapidly growing sales from 14% in 2022 to 7.5% in 2026. It is then maintained at a constant 7.5% of revenue. Total R&D expense (including employee costs) is estimated to reach 15% of revenue based on benchmarks, assuming a 50:50 employee:non-employee split.
3. Investment in Facilities: Investment in facilities is assumed to resume at a constant 5% of revenue per annum. In practice, it is likely to be much lower during FY23/FY24, but eventually additional facilities for growing sales and growing product variants will be required.
4. Corporate, Overheads and Other Expenses: Operating leverage means these fall as a % of revenue from 25% in FY22 to 10% in FY32. They are assumed to scale at 60% of the rate of revenue growth each year. (They were lagely flat from FY21 to FY22, but this cannot be sustained, and is considered to be as a result of resource discipline to avoid a capital raising.)
5. Other assumptions
- Effective cash profit tax rate of 25%
- Discount rate 10%
- Inflation 2.5%
- No debt. (Modest gearing will deliver further upside to valuation)
Key Assumptions - Scenarios-specific
Product uptake
A key uncertainty once accounts are established is the organic growth within an account as surgeons use the product across a wider range of indications, and as new products are added to the portfolio in the longer term (Note: this is not "blue sky" as they already exist). Rather than model different indications and applications (e.g., diabetic foot ulcer), a generic factor is applied to the average sales per account to model this growth. Annual in-account growth modelled is:
- +5% p.a. Bull Case (+62% compounded to 2032)
- +3% p.a. Bear Case (+34% compounded to 2032)
These numbers appear conservative given the statement by Max Johnston in the FY22 Results call that accounts existing at the start of the year saw on average of 88% growth. Clearly, this level of growth represents an adoption curve that must flatten off. In any event, the modelled scenarios are likely highly conservative. However, it is equally important not to double-count the two-year learning curve for the sales and marketing team effectiveness.
The combine effects of i) sales and marketing effectiveness and ii) in-account growth (use, indications, products) means that the average annal sales per S&M employee grows from $0.80m in 2024 to $1.34m in 2032, expressed in $2022 for compariso (Bull Case)n. This is reasonable in that the top performers are already achieving $2m p.a. and recognises that within any salesforce there is a wide range of productivities per employee.
Margins
$PNV currently reported continued improvement of gross margins to 95%, assisted by high volumes and increased plant utilisation.
It is hard to make reasonable assumptions for margins into the future, however the following are considered:
- As product development continues, the product portfolio will become increasingly complex lowering GM
- As the sales mix broadens to include more sales outside North America, averaged realised prices will fall
- As synthetic and biologics continue to displace traditional therapies, product-to-product competition will increase
- Attractive margins and expiring patents will lead to entry of alternatives
- Increasing market footprint will require higher inventories
- It may be more challenging to execute a direct sales model and deals via distributors have lower margins.
Over the modelled period margins are assumed to decline by 0.5% p.a. every year in both the Bull and the Bear case, driven by different factors. In the Bull case, cost of complexity and market mix are the dominant factors. In the Bear Case, greater reliance of distributors and competition are the major factor.
Market Penetration – sense-checking model outputs
2032 sales of almost $900m are sense-checked against 2020 figures on TAM and growth provided by $PNV for dermal repair, reconstruction, and hernia markets. Assuming lower market CAGRs of 7.5% than those projected by $PNV to provide a significant margin of safety, the project Bull Case modelled sales represent the following market shares: dermal scaffold 15%, reconstructions 3%, hernia 2%, with correspondingly lower shares in the Bear Case. There is a significant upside if synthetic implants become the standard of care displacing both traditional methods and biologics. Neither are assumed in the Bull Case. Drug elution remains a blue sky upside, as significant sales from this are unlikely to arise during the period modelled given longer regulatory approval timeframes, and are account for in part by the growth rate in the Continuing Value period (See below).
Continuing Value Growth Assumptions
Both Bull and Bear scenarios assume that $PNV continues to establish itself as a leading, global wound care company, continuing to invest in innovation to drive growth beyond 2032. Continuing period growth assumptions are a significant drivers of valuation. The two modelled assumptions are:
- Bull Case: 7% p.a. growth ahead of growth in global healthcare, given by continuing market penetration outside developed markets, with new products driving penetration of the reconstruction and drug elution markets
- Bear Case: 5% p.a. growth in line with mature, leading healthcare companies. $PNV like remains focused on dermal treatments. (Limited success in reconstructive surgeries and drig elution).
Conclusions
The Bull and Bear cases are both intended to represent reasonably probable cases given everything we know today.
On the downside, major mis-steps such as a botched acquisition, product quality issues or emergence of a new, superior technology are not considered.
Equally, on the upside there is ample room for stronger growth cases. For example, Swami is looking for a bolt-on acquisition that put 100 people on the ground with access to the US podiatric surgery market. If acquired at a reasonable multiple, the revenue synergies could be material over a few years.
Some key indicators to be tracked over the next 2 years, to update the model mechanisms include:
- Gross margin evolution
- Build out of sales and market organisation
- Account growth and $ sales per S&M employee, esp. in USA
- Mix of direct to indirect S&M models (The Canada experience is key)
- Market share (Continue to track Integra and Aroa)
- FDA approvals for new indications; progress of clinical trials; publications
- Evidence of trajectory in most mature markets (ANZ)
Comparison to the last valuation
The major difference between the current valuation and prior model, is that the latest model explicitly recognises the challenges of building out a global S&M organisation. This is now considered the key value driver, particularly given the sluggish growth achieved in Europe via the distributor model. More generally, growth assumptions have been toned down by explicitly considering more of the factors that can slow progress - arguably better representing real life!
Disclaimer:
This is not financial advice. Modelling is for author's personal use only and illustrates hypothetical scenarios. No undertaking is given that model is free of errors. Do not use as the basis for investment decisions.
We are still holding Polynovo shares IRL and feeling a little pain with our shares down 15%. However, if Macquarie’s target price is anything to go by, we shouldn’t be too concerned at all!
Macquarie is forecasting 2H25 revenue of $71.7m, and will be looking for management commentary regarding its BARDA trial and performance of its new MTX product when it releases its upcoming results.
Macquarie currently has a 1 year price target of $2.80 on Polynovo shares suggesting a 130% upside over the next 12 months (https://www.fool.com.au/2025/07/21/broker-tips-these-3-asx-small-cap-healthcare-stocks-to-rise-56-79-and-130/).
Macquarie is much more bullish than most analysts with the 1 year price target consensus from 9 analysts on Simply Wall Street at $2.02. Analysts are forecasting EPS growth of 33% and ROE of 23% over the next 3 years.
Held IRL and SM
$PNV's requested a halt as it is about to announce a "significant collaboration" with beta-cell technologies in Europe.
I'm assuming this could be a drug elution product that would use Novosorb as some kind of implant/drug elution carrier to use in concert with one of Betacells drugs? Drug elution technologies were always in prospect at $PNV, but we haven't heard much about it for a while.
Despite the trading haly, I am assuming that this would have to be proven through a full clinical trial program. My assumption is that it is early stage. But I am only guessing here.
There's nothing I can see on their latest pipeline chart (below from Macquarie Conference)

Polynovo ($PNV) announcing this morning that CEO Swami Raote will be finishing up in June but stepping down immediately.
Obviously discussions between the Board (specifically David Williams) and Swami didn't go well.
Very odd announcement, entitled a repsonse to an article in The Australian, rather than what it appears to be .... CEO Swami's intended resignation, at the request of the Board (DW?).
Very odd indeed.
The language makes it clear that agreement on the separation has not yet been reached. So is the announcement a device to force the end?
As some of us have speculated here, given the rapid fall in growth rate including the lack lustre progress in India, it was looking increasingly likely that Swami's incentives are likely out of the money, and that his retention was also a potential issue. Now it looks like that rather than jumping, he is being pushed.
Margin call gave a different lens on this issue, focusing on the behaviour of Chairman David Williams.
Whatever the reality and the truth, it appears all is not well with management and the Board at $PNV.
Disc: Not held
https://investorpa.com/announcement-pdf/20250307/111468.pdf
7 March 2025
ASX Announcement
Response to article in 'Margin Call'
PolyNovo Limited (ASX: PNV) refers to the article in this morning's 'Margin Call' column in The
Australian newspaper.
PNV confirms that on Friday last week there was a confidential discussion with the Chief Executive
Officer, Mr Swami Raote, requesting his agreement to cease his employment with PNV effective
June 2025 at the end of his contractual notice period and to step down as Chief Executive Officer
effective from an earlier date. The discussion proposed terms for his separation if agreement was
reached and revealed that non-executive director, Dr Robyn Elliott, would be stepping into the role
as Acting Chief Executive Officer pending the appointment of a permanent replacement.
This announcement has been authorised by the Board of PolyNovo Limited.
About PolyNovo®
$PNV published their HY result, with the call starting in 3 minutes.
A few quick observations from me, not going over old ground that we've covered here before:
- Expense control OK, with Opex up +15.4% cf. Revenue +22.8%
- Operating Cashflow strongly negative .... why is this?
- Modest NPAT increase up to $3.338m compared with $2.694m in PCP
- New products still at the pre-clinical phase, with R&D spending modest
- New facilities on track.
Overall impression - nothing here causing me regret on my exit decision. I still think its trajectory has slowed too quickly for this to represent a compelling investment proposition. I don't have a view on fair value based on these numbers. But my model is shot, as is my thesis.
Here's a complete summary courtesy of my BA ChatGPT (I've had a quick look - it seems OK, but I've not vetted it in detail, so beware!)
PolyNovo 1H FY25 Performance Summary (Compared to 1H FY24)
Summary
PolyNovo delivered a strong first-half FY25 performance, marked by record sales, improved profitability, and strategic advancements in product development and market expansion. While operating cash flow turned negative due to increased investment in growth, the company remains well-funded, with A$30.5 million in cash reserves. With continued investments in manufacturing, R&D, and clinical trials, PolyNovo is positioning itself for sustained long-term growth.
Financial Highlights
- Total Sales: A$54.1 million, up 28.1% from A$42.2 million in 1H FY24.
- Total Revenue (Including BARDA): A$59.9 million, up 22.8% from A$48.8 million.
- Net Profit After Tax (NPAT): A$3.3 million, an increase of 23.9% from A$2.7 million.
- U.S. Sales: A$41.2 million, up 27.9% from A$32.2 million.
- Rest of the World (ROW) Sales: A$12.9 million, up 28.6% from A$10.0 million.
Cash Flow Highlights
- Net Operating Cash Flow: -A$12.5 million, compared to A$0.6 million positive in 1H FY24.
- This reflects increased payments to suppliers and employees (A$63.9 million vs. A$45.3 million in 1H FY24).
- Receipts from customers: A$44.3 million, up from A$41.1 million.
- Receipts from BARDA reimbursements: A$7.3 million, up from A$5.1 million.
- Net Investing Cash Flow: -A$4.5 million, compared to -A$0.5 million in 1H FY24.
- Driven by increased capital expenditure of A$5.1 million (up 361.2% from A$1.1 million) for R&D and new facilities.
- Net Financing Cash Flow: -A$2.2 million, compared to -A$1.4 million in 1H FY24.
- Repayment of borrowings (A$1.9 million) and lease liabilities.
- Cash & Cash Equivalents (End of Period): A$30.5 million, down from A$45.9 million at June 2024.
- Key Driver: Increased investments in infrastructure, R&D, and sales expansion.
Operational and Strategic Developments
- NovoSorb MTX Launch: Achieved A$2.1 million in sales following a successful U.S. launch in Q4 2024.
- Pipeline Expansion: Introduced new sizes and thicknesses for NovoSorb BTM and NovoSorb MTX.
- Hernia Repair & Plastic Mesh Development: Pre-clinical stage initiated for new medical applications.
- Full-Thickness Burns Clinical Trial (BARDA Supported): Achieved enrollment of 120 patients, paving the way for FDA engagement on potential paediatric burn treatment.
- Manufacturing Expansion: Construction commenced for a new facility in Port Melbourne (Operational by December 2025).
- Innovation Centre: Finalised design, with opening planned for June 2025.
Key Financial Metrics
- BARDA Revenue: A$5.4 million, up 10.2% from A$4.9 million.
- Capital Expenditure: A$5.1 million, up 361.2% from A$1.1 million, reflecting investment in R&D and infrastructure.
- Research & Development (R&D) Expenditure: A$5.1 million, up 3.8% from A$4.9 million.
- Employee Growth: Workforce increased to 282 employees, up 19.0% from 237 in 1H FY24.
Market & Growth Strategy
- Market Penetration: Focus on expanding U.S. market share, taking business from established competitors.
- Rest of World Growth: Increased procedure and market development efforts in Europe, the UK, and Ireland.
- Innovation & Expansion: Developing solutions for trauma, reconstructive surgery, and general surgical applications.
Disc: Not Held
A good friend of mine is a plastic surgeon in Sydney and I asked them if they had used PolyNovo's NovoSorb.
They said:
"Have extensive experience
Only works 50% of the Time
So sometimes a get out of jail card
But frequently disappointed so not the magic bullet that will revolutionise recon.
I wouldn't invest if that's what you're asking"
Poynovo (ASX:PNV) announced to the market that it had a record monthly sales of $A10.1m (unaudited) for November 2024.
Highlights:
• The U.S. business grew strongly, with monthly sales of $A7.0m (unaudited) and YTD November sales of $A33.9m are up $A7.3m or 27.3% on STLY.
• Rest of World had monthly sales of $A3.1m (unaudited) and YTD November sales of $A10.5m are up $A2.0m or 24.0% on STLY. After attaining market leadership in UK and Germany in full thickness burns, we continue to disrupt the market by expanding into soft-tissue reconstruction use cases in UK and Germany by displacing the current gold standard in oncological excisions and surgical dermatology.
• Total group revenue for the month, including BARDA was $A11.0m (unaudited), and YTD November group revenue of $A49.6m is up $A10.1m or 25.4% on STLY.
Particularly pleased with the growth in Rest of the World (ROW) Monthly sales.
David Williams providing milestone monthly sales results will serve well enough for interim updates.
Keen to see how NovoSorb MTX uptake continues from here.
Will continue to hold both in RL and here on SM.
Just in case you missed @Jimmy’s News Summary DJ Australian Equities Roundup -- Market Talk 26 Nov 2024 15:01:13 straw:
2311 GMT - Morgans senses a buying opportunity in skin-healing tech developer PolyNovo. The stock has fallen roughly 25% since the end of September, which analyst Scott Power thinks is partly because PolyNovo didn't give a trading update at its annual meeting of shareholders. "Consensus has revenue growth of 29% for FY 2025 which will be skewed to the 2H and we are confident it can be achieved," Morgans says. "Growth of 20%+ is expected to continue in FY26/27 driven by regional expansion as well as additional indications." Morgans has a A$2.85/share price target and add call on PolyNovo, which ended Monday at A$2.04. ([email protected]; @dwinningWSJ)
Those following $PNV will no doubt be aware of the production problems competitor Integra ($IART) has faced following product recalls from the key Boston manufacturing facility. I got my BA (Perplexity.AI) to do a run down of the 4th November $IART analyst call.
Key message is that, while the problems have had a significant impact over the last year, $IART seems to be on the way back.
Clearly, in a market with a range of competing options in dermal repair, some surgeons facing supply problems will have been forced to switch. It will be interesting to see whether they switch back.
The next couple of quarters will likely be positively impacted by restocking orders, so over the coming quarters I'll need to analyse the Tissue segment sales across mutiple quarters to look through that.
It should also provide some insight into the overall progress of the segment, via comparisons with $AVH, $ARX and $PNV. While these companies have products that are not equivalent (there are both overlaps and complementarities!) a key question I am trying to get a better handle on is the respective growth contributions from 1) expansion of the market segement within established indications, 2) expansion into new indications, and 3) market share gains. My deep dive into this segment a couple of years ago (published here) made some assumptions about the market depth and breadth in the US. It's time to revisit that analysis, ahead of the learning about $PNV's US sales in February.
Here's the detailed analysis of the transcript
-------------
Integra provided several key points regarding its Tissue Technologies segment and recent production issues:
Tissue Technologies Performance
- Tissue Technologies sales were $110 million in Q3 2024, down 3.6% on a reported basis and 3.7% on an organic basis compared to the prior year.
- Excluding the prior year impact from the return of product manufactured in Boston, organic growth was down 9.4%
Production Challenges
- Production shortfalls for Integra Skin limited the company's ability to fully meet demand
- These production issues were the primary cause of the decline in Wound Reconstruction sales
Recovery and Outlook
- The company has been ramping up production of Integra Skin through the third quarter
- They expect to return to historical revenue run rates for Integra Skin in the fourth quarter of 2024
Other Tissue Segment Highlights
- Despite the Integra Skin challenges, there was strong growth across other parts of the Wound Reconstruction franchise:Low double-digit growth for DuraSorb
- Low double-digit growth for MicroMatrix and Cytal in the UBM platform
- Overall high single-digit growth in the UBM platform
- Private label sales were up approximately 13% compared to last year due to favorable order timing
International Impact
- International sales in Tissue Technologies were down mid-double digits, primarily due to the Integra Skin production shortages
The company appears to be addressing the production issues and expects improvement in the near future, particularly for Integra Skin products.
DW was interviewed this morning by John Hester, Bell Potter (Healthcare Analyst) at the Bell Potter Heathcare conference. No slides or significant information, apart from one. John took David head on about the lack of update or guidance at the recent AGM.
David explained that the Board is comfortable with analyst consensus, and if they weren't - either up or down - they would update the market. At the same time, he said they will not give forward guidance because, with their high growth, doing that is a pathway to trouble (I agree!)
So, the best view is that $PNV is on track to achieve about 28% revenue growth in FY25, with consensus at $134.5m. (My source: www. marketscreener.com. n= 9)
Disc: Held in RL and SM
Here are the Chairman and CEO speeches for the $PNV AGM to start in a few minutes.
NO trading update! (There should at least have been a 1Q revenue update, as they have an established record of updating even if irregularly - what are they playing at?)
Under their continuous disclosure obligations, if the sales information was materially different to what the market is expecting, then you'd have expected an announcement. So, at this stage I am assuming revenue is in-line.
Indeed the statement in Swami's speech "The US remains the driving force for our company, growing by 49% over prior year. As we expand into other trauma, infection and active complex wounds, the number of patients healed is expanding at a stronger pace."
"Expanding at a stronger pace" is definitely ambiguous, but it implies that the rate of sales growth is not slowing.
It will be interesting to see if there is an update after the meeting? There will certainly be Q&A on this topic!
(I cannot quiet the voice inside that says this level of obfuscation is deliberate.)
Disc: Held
Those who follow me here and also follow $PNV will know that I am NOT a technical investor.
However, I am always eager to learn, and so today plotted the chart below within my CommSec Ap. using the Bollinger band technicals views, which plots the Simple Moving Average (in this case 20-day) with the +/- 2 standard deviations. The basic idea being that when it flies above the upper limit, the stock is overbought and when is flies below, it is oversold. (I have no idea what other technical alogithms say ... @Saiton ... momentum traders are probably offloading?)
So, in this year's version of the $PNV rollercoaster, we've breached the lower Bollinger bound for the first time in over a year.
The other thing I'd note is that the volumes have been modest for a while now, in part because the shorts have quietened down quite a bit on this stock.
Let's overlay newsflow on that:
- 8th May - Record Month
- 23rd July - FY Trading Update (a good news story)
- 16th August - FY Presentation (confirm in the details of the good news story)
- 4th September - Pivotal Trial Complete (quite a good news story)
- 18th Sept and 2 Oct - Director Selling (not good news)
- Number of days since material good news: c. 95
Make no mistake, $PNV is still a highly-valued company, based on the fundamentals, and riding the rollercoaster is a condition of being a shareholder. But I think this is primed well for the next update.
So What?
I don't buy or sell on technicals, I only do that on valuation. And my current valuation is $2.60 ($2.30-$3.50). My problem is, that $PNV is already my largest RL holding. But it has fallen so far below my lower limit of valuation that it sure is tempting to take a small bite, particularly given that I am well within my maximum single stock exposure on a cost-base basis.
Decisions, decisions.
Disc: Held in RL and SM

$PNV have today announced the conclusion of the patient enrolment phase of the BARDA trial (PIVOTAL CLINICAL TRIAL), which started (I recall) in late 2021.
It has taken some 3 years to enrol 120 patients with full thickness burns (FTB) sufficient to meet the criteria, although the recent addition of India as an enrolment location has significantly accelerated the conclusion of the trial enrolment process.
It's not clear what the forward timelines are. Presumably, all patients have to progress through treatment to their end point. In the case of FTBs, I understand the primary end point is wound closure after 12 months. If it is required that all patients have to reach this end point, then there would be at least another year before submission of the data, and then around a further year for a Final FDA decision.
However, given that $PNV have repeatedly referred to ongoing dialgoue between BARDA, the FDA and $PNV, and given that many of the early patients will have passed the end point, as well as the repeated references to this in the last three investor presentations, I anticipate that the PMA decision may run to a shorter timeframe.
$PNV have been very disciplined not to say anything about timeframes (although DW always sails close to the wind on this), because ultimately, neither $PNV nor BARDA are in control of this. That said, given that BARDA is a government agency, then that probably counts for something.
Essentially, a FTB on-label indication will likely 1) lead to a BARDA stockpile purchase and 2) further accelerate adoption for FTBs in the US and 3) facilitate approval for FTB in those countries where FTB is not yet an approved indication. Note: it is used for FTB in many countries, and is already used for this off-label in the US.
Given all of this, the announcement is not price sensitive.
That, at least, is my understanding. Happy to be corrected by any StrawPeople who know better.
Disc: Held in RL and SM
Courtesy of DW's mail round, here are some of the analyst responses to the $PNV result:
Bell Potter: TP from $2.52 to $3.00; upgrade to BUY
Morgans: TP from $2.50 to $2.85; retained as ADD
Macquarie: TP from $2.75 to $2.85; retained as OUTPERFORM
Evans & Partners: TP $2.65; Positive
Wilsons: No TP - First Look only; OVERWEIGHT
OVERALL: Modest increases in TP overall. Generally positive
Given that DW has signalled the potential end of "record month" reporting, then 1H25 is the next catalyst as, in my view, the FY25 revenue growth assumption of the consensus is undemanding ($133.3 / $104.8 = +27%), which I expect will be readily surpassed even given a potential "BARDA" effect.
Disc: Held in RL and SM
Following the FY24 results, I have update my valuation for $PNV.
Result: $2.60 Range = ($2.30 - $3.50)
Method: 10 yr DCF
Key Assumptions:
- BTM/MTX only
- Market growing at 8% CAGR
- Assumes market share grows from 2.7% today to 15%/19%/22% by 2034 (Integra estimate of $2.5bn TAM burns & trauma)
- New products beyond BTM/MTX platform treated as a sensitivty to the Continuing Value Growth 5% instead of 4% base.
- %GM declines from 94% (2024) to 88% (2034) due to increasing mix of 1) developing markets, 2) complex product mix, and 3) large increments of capacity that will be under-utilised from time to time
- Capex to deliver just in time capacity: 1st $500m for $27m, 2nd $500m for $40m
- Range of expense/revenue ratios modelled from FY24 to reach reasonable benchmark for global medical device company by 2034.
- WACC 10%; Tax=30%
- Other minor assumptions for interest and working capital scale with revenue
Comments
Compared with my last detailed valuation from Sept-22, I consider the FY24 result puts a much firmer base under the low case.
@Parko5 my top end just hits your $3.50. This is worthy of a comment. There are plausible scenarios where I can get up to $4.00 without stretching my own belief, and so it's worth looking at what's not included (see section below). You'll also see that I contradicted myself a little about revenue growth in FY25. I do indeed model values ranging from 39% to 58%. The reason is a modelling convenience, as the market share increases linearly from today and I didn't break out BARDA. In practice, the "BARDA Effect" that I mentioned in the earlier post is a transient 1-year thing that hits 2025 disporportionately (I'm assuming). It has little consequence in the overall valuation, so I chose to ignore it, as my model is complex enough without adding more complexity!
What's Not Included
Upsides
1. BARDA full thickness burns approval by FDA in 2025, driving "higher for longer" growth rates
2. A strongly favourable IQVIA report in 2025/26 - showing economic and patient outcome advantages of Novosorb, leading to wide adoption by HMOs etc and accerlating market shift away from biologcs More "higher for longer".
3. Award of multiple Federal or State Government Tenders in India, leading to India rapidly taking off
4. Early entry to China beyond Hong Kong (pre-2027)
5. New Products (i.e., beyond BTM and MTX variants) commercialised before 2028 and achieving significant revenues before 2030.
Downsides
6. Emergence of alternative synthetics competitors eroding market share gains in the later years
7. Loss of momentum due to Integra-like product recall (These things happen in medical devices!)
1.& 2. can be argue to be contained within the high revenue growth assumptions. However, the impact could be more material increasing the 2034 market share by accelerating switching from competing treatments. They would also likley accelerate global adoption, particularly in countries without capacity to conduct their own trials.
Model Outputs and Inputs
(I can answer and provide more detail on methods used to get to each of the items below, if anyone wants.)
1. Valuation
 2. Model Output Table
2. Model Output Table

3. Input Tables

"i" related to FY24; "f" related to FY34 - model shows linead trend in expense ratios across 2024-2034

Disclaimer: This is intended for my personal use only. It is not advice and must not be used as the basis of an investment decision.
I'm limbering up for the DW Show at 2pm this afternoon with a quick review of the $PNV results. The financials have been well-telegraphed in advance, and I've gone through the Accounts and it's all remarkably close to my forecasts.
FY24 Results and Financial Statements

Their Highlights
- Total revenue including BARDA of A$104.8m, up 57.5% on STLY of A$66.5m
- Strong growth in U.S. achieving record sales of A$68.7m up 49.0% on STLY of A$46.1m.
- ROW sales of A$23.4m up by 73.3% on STLY of A$13.5m.
- Positive cash flow from operations of A$3.7m up 155.7% on STLY (A$6.6m)
- Net profit after tax of A$5.3m (FY23: A$4.9m loss)
- At year end the business had A$45.9m cash and cash equivalents
During the Period, the Company’s other key initiatives and achievements include:
- Record monthly sales in April of A$9.2m (monthly revenue: A$10.5m) and May of A$9.8m (monthly revenue: A$11.3m).
- Initiated a full market launch campaign in the U.S. for NovoSorb MTX in June 2024.
- Strengthened the U.S. team from 93 to 107 (June 2024)
- Increased U.S. customer accounts by 197 from 299 to 496 (June 2024)
- Increased global employee headcount from 218 to 254
- New C-suite roles - Chief Medical Officer, Chief People Officer, General Counsel and President, Asia Pacific.
- Supplied into certain war zones for humanitarian needs.
- Obtained registrations for Bolivia, Ecuador, Thailand, and Sri Lanka.
- Enrolled 120 patients into the U.S. BARDA pivotal trial for full thickness burns.
- Finalised design and selected a builder of the third manufacturing facility to service up to $500m in additional revenue.
- Awarded Victorian Government grant of A$2 million for R&D facilities expansion, subject to customary conditions.
My Analysis
The key for today is that $PNV delivered on their commitment at the capital raise to be profiable in FY24.
EBITDA, EBIT, NPAT, and FCF all positive. The first year we've had this.
Gross Margin % of 94.8%
In the US, with modest sales team growth, new accounts and revenue grew strongly, reflecting the lag effect of 1-2 years between adding headcount and driving revenue per account. A key question is where is the US trajectory from here?
2024 has been a foundational year: 1) broading market approvals across the global where Novosorb can be sold 2) progressing the design of the major manufacturing capacity expansion, 3) re-igniting R&D to build out further products to exploit the platform technology (R&D expense in FY24 up to $11m from $7,4m, but still only a CSL-esque 10% of revenue), and 4) building out the management team. These are all important steps in building a business from this start-up with a genuous product.
In the Chairman and CEO remarks, there were further details on revenue progression in key markets:
- UKI up 81.5%
- EU/Germany distributor markets up 81.2%
- Australia up 38.7%
I take the UKI as a good indicator of what the EU can ultimately do, and it looks like the distributor is kicking into gear. UK/EU growth will be important in maintaining the group trajectory as the US inevitably matures.
The addition of licensing in SUPRATHEL means it looks like $PNV are taking a leaf out of the $AVH book. Once you have the sales foot print, who need to give them more things to help drive contribution margin per account. So, good.
What's not mentioned - India. There is a lack of granularity there. So hopefully the analysts will try to tease out some more on that on the call. After all, we've had 20+ people now working that market for over a year, and there have been some qualitative stories of progress. India does not need to be a "today thing", however, over the longer term the potential for the product to get traction at a reasonable contribution margin in middle income/developing markets helps the long-term growth thesis. (And after all, it's why - or one reason why - Swami joined the company!)
My Key Take Aways
Report entirely as expected. No surprises. So the question is what else can we learn on the call at 2pm.
Lunch now, and then I'll be sure to get my ringside seat for the DW show!
Disc: Held in RL and SM
$PNV is presenting at the Canaccord Genuity Growth Conference.
There's no market sensitive or significant news we haven't heard before, but we don't always get the information in the most cohesive manner (!!), so this might be of interest to some. The presentation pulls everything together quite nicely.
What has been interesting in the last two conference presentations, is that the portrayal of new products and timeframes has re-emerged, after an absence of over three years. The new head of R&D has been in place for over a year now, so this makes sense.
Looking forward to the FY results - not expecting any surprises or material new information, but hoping for a little more granularity on how some of the RoW markets are tracking. Also, I'm looking to see if there has been any signficant change on capex, not that the costs for the new facilities must be in.
With RoW at $23.3m sales and 73.1% annual growth, that's ahead of where all of $PNV was in 2020, when it was growing at 54%! Just reflect on that.
Disc: Held in RL and SM - my largest position in both
To start a standard bull base for me - given revenue for 2024 of $104m and assuming a 25% CAGR to 2029
=> 2029 Revenue: $316m
Assuming GP% drops to 75% (due to the majority of sales in developing countries), $77m opex this year and a 10% CAGR in opex going forward to $124m
=> 2029 EBT: 114
Assuming 360m shares (modest buy back over time)
=> 2029 SP: $5.18
discounting at 10%
=> 2024 Share Price: $3.20
What if I wanted to get hyper-bullish? A different approach:
According to the Macquarie Presentation, they will have capacity to produce 680k units by 2027
Lets assume they are selling at 80% of that capacity in 2029: 544k units
In 2023 they produced 38k at a total cost of $12m (Revenue - GP; 65 - 53)
=> Cost per "unit": 315k
Lets assume costs remain the same (productivity improvements offset inflation in costs)
=> 2029 COGS: $167m
Assuming the same 75% GP margin
=> 2029 Revenue: $666m (ooh, spooky, maybe DW is Satan not Santa)
Let's assume it is gonna take a big sales team to get there so Opex increases at 30% CAGR from 2023 levels to $280m
=> 2029 EBT $220
with 660m Shares
=> 2029 SP: $10
=> 2024 SP: $6.20 (10% Discount)
For now, for now I'll stick with the first approach, but nice to have a possible upper limit if things start to get crazy.
I have no opinion on the accuracy, but thought I’d throw it out there if people would like to compare assumptions that go into the valuation:
Our fair value estimate for Polynovo is $1.05 per share and assumes the company is profitable from fiscal 2024 onward.
We forecast a five-year group revenue compounded annual growth rate ("CAGR") of 26% forward to fiscal 2028 versus a trailing two-year revenue CAGR of 50%. This is driven by Polynovo’s key US geography where we forecast a five-year revenue CAGR of 21% for the region.
We expect the firm increasing its sales staff to support market share gains. In addition, we expect the recent product launch of NovoSorb MTX to increase penetration within existing hospital accounts given its broader applications. Our forecast five-year revenue CAGR for geographies outside the US is 40%. This is driven by recent entries in India, France, Spain, Canada, and Hong Kong, as well as planned entries into China and Japan. As such, we forecast revenue contribution from the US to drop to 65% of group sales in fiscal 2028 from 79% in fiscal 2023.
On the profitability front, we expect group midcycle operating margins to settle at around 35% by fiscal 2033. We forecast Polynovo’s maiden profit in fiscal 2024 and margin expansion from operating leverage. Our estimates deliver EPS growth of 18% at midcycle. We forecast average annual capital expenditures of roughly AUD 6 million over the next 10 years, or 3% of group sales. We also factor $25 million in capital expenditure over fiscal 2025 and fiscal 2026 to fund a new manufacturing facility which has capacity to service an additional AUD 500 million in annual sales
DW has just circulated two analyst notes to everyone on his email list. Macquarie (who are maintaining their TP of $2.75 and Outperform) and Wilson (who are at $2.65 are Overweight). Neither are updating recommendations and I assume are awaiting the FY, with its forward looking statements.
My reason for this Straw is that the Wilson report contains some interesting insights, both on the US and also on PMI the distributor in Europe - who is running a full thickness burns trials. The commentary is rather encouraging and succinct, so I've extracted the relevant paragraphs.
From Wilson's Report
"Polynovo has pre-released its unaudited revenue results for FY24. US sales increased 49% to $104.8M; with ROW sales up 73% to $23.3M. US performance in 2H24 was 8% lower than we forecast; offset by a 10% beat from ROW businesses plus grant support from BARDA. We’re not allowing a $3.5M sales ‘miss’ in USA dissuade us from our O/W thesis on PNV. Burns still constitutes ~68% of product volume and is invariably lumpy. Anecdotally, PNV was winning up to 70% market share by surface area in key US centres over 4Q24, thanks to its competitor’s protracted difficulties (Integra’s recall). We assess that >30% of the absolute US growth dollars in FY24 has come from pricing, with more to come, as described in our recent upgrade. The ROW business stands to benefit in FY25e as well, with several jurisdictions set to re-tender business"
"The absence of Integra’s PriMatrix and SurgiMend in the marketplace created a $30-40M annualised revenue opportunity in burns, trauma and reconstructive surgery. Notwithstanding hefty price increases, BTM remains super-competitive on (per cm2 ) pricing and enjoys a broad clinical following. Feedback suggests BTM has usurped Integra now on a volume basis (at least in some major ABA accredited burns centres) and carries that incremental share tailwind into FY25e. Internationally, we understand that PMI’s markets (distributing into Germany, Austria, Switzerland), Spain and Turkey starred. BARDA revenue support was also higher than forecast, given how quickly Polynovo’s PMA-directed trial in full-thickness burns has enrolled."
"Forecasts under review. At this stage we see little change to revenue forecasts, expecting continued market share gains (both organically in burns and at Integra’s expense), incremental volume via extension into trauma indications and flagged pricing momentum. Thinking about costs leading into FY25e, we’re cognisant of a few (positive) areas where investments may be drawn forward (e.g. SKU expansion for MTX, building on early product traction in trauma; and an earlier PMA filing in relation to full-thickness burns)."
Disc: Held in RL and SM
I'm not sure how much notice the market is paying to Broker/Analyst views for $PNV, however, I wanted to share some analysis which I know some of us will find interesting.
My valuation
First, I want to state that I won't be updating my own model until after the FY results are out. But for the record, my valuation is $2.37 (if I adjust my $2,25 from Feb-24 by a further manual adjustment of +5%)
My last full evaluation across a range of scenarios from almost a year ago was: $2.16 ($1.63 - $3.63), which if I roll forward by one year becomes $2.37 ($1.80 - $4.00). \
Now when I update the model in late August, I'll have to re-run the growth scenarios, have an updated cost structure and view on capex. So, it could end up looking quite different. My sense, however, is that the range will narrow, and that the low end of the range will come up.
For those of us who who might respond with "what good is a valuation with such a wide range?", my answer is that it represents unresolvable uncertainty. Remember, $PNV is still passing through the inflection point so, good luck justifying a tighter range.
Brokers Consensus
I use two sources when I look at brokers/analysts:
- Marketscreener.com: $2.18 ($1.00 - $2.75); n=8
- Tradingview.com: $2.46 ($2.00 - $2.75); n=5
I'm going to ditch the low value on marketscreener.com of $1.00, because I think it has no credibility. That moves the marketscreener.com average TP to $2.34 ... closer to Tradingview. (Note: on these services I can't see the individial datapoints)
Because of my concern in this case about the marketscreener.com dataset, I am going to focus the rest of the analysis on the analysts in tradingview.com.
So, based on today's close of $2.60, the market is now only about 6% ahead of the analysts.
Revenue Growth
The insight I wanted to share, is that over the next 6-9 months there is a chance for a material re-rating of $PNV.
I'll make the case by focusing on revenue growth - because it is still the dominant factor.
The analyst "consensus" for revenues are as follows, with % growth yoy in parentheses:
FY24: $104.8m (+57.5%)
FY25: $133.5m (+27%)
FY26: $167.8m (+26%)
Of course, it is reasonable to expect revenue growth to start tailing off at some point, but if you consider the last 3 annual y-o-y growth rates of FY21 (32.0% - COVID access impact) FY22(42.8%) FY23(58.8%) FY24 (57.5%), something dramatic would have to happen in FY25.
However, we know that:
- The category is expanding
- BTM is taking market share
- MTX is now also being rolled out
- The global rollout is still firing on all cylinders
My Conclusions
I'm not a seller of any $PNV much below about $3.80.
Of course, it will continue to be volatile. But the risk of getting off the bus and then being unable to get back on with this one is just too great for me.
I believe this is going to get more focus from the market as we move through the inflection point - i.e. in FY25. Of course, I realise that - for a couple of years at least - some "talking heads" will shake their heads at eye-watering P/Es. But that is an irrelvant measure AT THIS STAGE.
I'm very interested to see what upgrades come through for FY25 and FY26 over the coming 6-9 months, and everything that entails.
If I wasn't at a full allocation, I'd still be a BUY today.
Finally, I said in another straw recently, that I would test all valuations in healthcare through an M&A lens. I have not yet done that for $PNV. I will in August.
Disc: Held in RL and SM
Especially for you @Rick !
Their Headlines:
• Total revenue including BARDA of A$104.8m up 57.5% on STLY of A$66.5m.
• FY24 sales of A$92.0m up 54.5% on STLY of A$59.6m.
• Strong growth in U.S. sales of A$68.7m up 49.0% on STLY of A$46.1m.
• ROW sales of A$23.3m up by 73.1% on STLY of A$13.5m including strong performances in developed markets like UKI, Germany and ANZ.
• Surgeon education and charitable contributions, widely used to support patients in conflict zones.
My analysis
Revenue is bang on where I expected. I had them coming in anywhere between $102m and $106m, which was easy to pick, given the absence of "record months" in the last few months.
All this should be good enough for them to hit their positive NPAT commitment for the FY.
I'm pleased revenue growth is holding up strongly. Analyst views have this starting to decline in % terms quite quickly over the next two years and, if they can hold it around +50%, then that's the opportunity for the next significantly leg up in SP, as that will drive a few years of very high EPS growth.
Conclusion.
On track. Holding for long term. My biggest RL position.
Although enjoying volatility is part and parcel of being a long term $PNV holders and, in that context, the price action of recent days is simply par for the course, I found the announcement below in a search of the news-wires. It came out last week.
It is not surprising that $PNV didn't make an announcement, as in my view it doesn't have a direct or material bearing on $PNV's sales, but it is good that $PNV is being sought out by innovators for collaborations.
Full text follows.
---------------------------------------
Spectral AI Announces Collaboration with Global Wound Care Company PolyNovo to Introduce DeepView System for Burn Indication to Australian Market
861 words
8 July 2024
12:00 GMT
GlobeNewswire
PZON
English
© Copyright 2024 GlobeNewswire, Inc. All Rights Reserved.
Spectral AI Announces Collaboration with Global Wound Care Company PolyNovo to Introduce DeepView System for Burn Indication to Australian Market
DALLAS, July 08, 2024 (GLOBE NEWSWIRE) -- Spectral AI, Inc. (Nasdaq: MDAI) ("Spectral AI" or the "Company"), an artificial intelligence (AI) company focused on medical diagnostics for faster and more accurate treatment decisions in wound care, today announced that it has signed a Memorandum of Understanding ("MOU") with global medical device company and burn wound therapy leader PolyNovo Limited ("PolyNovo") under which the companies will collaborate to assist Spectral AI in a potential limited deployment of its DeepView System for burn indication in Australia.
Under the MOU, PolyNovo will support Spectral AI's application to the Australian Special Access Scheme (SAS) with an ultimate goal of allowing Spectral AI to deploy up to two DeepView Systems at the Royal Adelaide Hospital and The Alfred Hospital in Melbourne to lay the groundwork for the Company's eventual commercial roll-out based on clinician evaluations and experiences.
The SAS was introduced by Australia's Therapeutics Goods Administration in recognition that there are circumstances where patients need access to certain medicines, medical devices, or biologics that are not already included in the Australian Register of Goods.
Spectral AI's DeepView(TM) System is a predictive device that offers clinicians an immediate and objective assessment of a burn wound's healing potential prior to treatment or other medical intervention. The image processing algorithm employed by the DeepView(TM) System utilizes multispectral imaging that is trained and tested against a proprietary database of more than 340 billion clinically validated data points. The DeepView(TM) System is non-invasive and cart-based, allowing for exceptional mobility within the healthcare setting.
PolyNovo develops and sells patented, bioabsorbable, synthetic, polymer technology used to reconstruct complex wounds, including deep dermal and full--thickness burns, and aid the body in generating new tissue. PolyNovo's FDA-approved NovoSorb(R) BTM (Biodegradable Temporising Matrix) and NovoSorb(R) MTX product portfolio is available in 37 countries around the world.
"PolyNovo's innovative therapies have proven to be life changing and it is one of the world's most respected providers of burn treatment solutions," said Peter M. Carslon, Chief Executive Officer of Spectral AI. "Understanding when it is appropriate to apply these therapies is paramount to realizing improved patient outcomes. We believe that the Day One wound healing assessment provided by the DeepView(TM) System empowers clinicians with the knowledge to make an informed and rapid diagnosis when time is of the essence. We are honored to work with an established market leader as we take these initial steps to familiarize clinicians in Australia with Spectral AI's technology, support their life-saving work, and help to elevate the level of patient care."
About Spectral AI
Spectral AI, Inc. is a Dallas-based predictive AI company focused on medical diagnostics for faster and more accurate treatment decisions in wound care, with initial applications involving patients with burns and diabetic foot ulcers. The Company is working to revolutionize the management of wound care by "Seeing the Unknown(R) " with its DeepView System. The DeepView System is a predictive device that offers clinicians an objective and immediate assessment of a wound's healing potential prior to treatment or other medical intervention. With algorithm-driven results and a goal to change the current standard of care, the DeepView System is expected to provide faster and more accurate treatment insight towards value care by improving patient outcomes and reducing healthcare costs. For more information about the DeepView System, visit www.spectral-ai.com.
Forward Looking Statements
Certain statements made in this release are "forward looking statements" within the meaning of the "safe harbor" provisions of the United States Private Securities Litigation Reform Act of 1995, including statements regarding the Company's strategy, plans, objectives, initiatives and financial outlook. When used in this press release, the words "estimates, " "projected," "expects," "anticipates," "forecasts," "plans," "intends, " "believes," "seeks," "may," "will," "should," "future," "propose" and variations of these words or similar expressions (or the negative versions of such words or expressions) are intended to identify forward-looking statements.
These forward-looking statements are not guarantees of future performance, conditions or results, and involve a number of known and unknown risks, uncertainties, assumptions and other important factors, many of which are outside Company's control, that could cause actual results or outcomes to differ materially from those discussed in the forward-looking statements. As such, readers are cautioned not to place undue reliance on any forward-looking statements.
Investors should carefully consider the foregoing factors and the other risks and uncertainties described in the "Risk Factors" sections of the Company's filings with the SEC, including the Registration Statement and the other documents filed by the Company. These filings identify and address other important risks and uncertainties that could cause actual events and results to differ materially from those contained in the forward-looking statements.
Investors:
The Equity Group
Devin Sullivan
Managing Director
Conor Rodriguez
Analyst
Their Product:
Novosorb - a biocompatible synthetic polymer used to create a dermal matrix, effectively scaffolding, to assist in the healing of the deeper layers of the skin (dermis). Used for burns and other wounds to fill and secure the wound as it heals, biocompatibility means the polymer is safely absorbed into the body as it heals.
2 versions of the matrix exist:
- BTM - consisting of Novosorb with a laminated outer layer to strengthen the foam and to seal the wound from external contaminants. The laminate has to be eventually removed as it does not biodegrade within the body.
- MTX - Novosorb without the laminate, can be implanted within the body and will be entirely degraded
Being synthetic Novosorb is a more consistent product (from batch to batch) in comparison to biologic equivalents resulting is less post-operative complications.
Their Customers:
In simple terms, based on who they receive money from, their customers are the medical institutions (public and private) that purchase Novosorb.
However, I think their actual customers are doctors and surgeons primarily in burns and trauma centers, but also other types of hospitals, medical centers and medical research institutes.
You could also say the patient that receives Novosorb as treatment is the end customer/consumer though.
To stich these all together:
Polynovos customers are medical institutions whose practitioners use Novosorb to treat patients. Practitioners will prefer Novosorb if:
- It is easier to use than other products
- Can be used in wider array of procedures
- Provides better outcomes for the patient
The crux of this is while the Institutions buy the product, it's the doctors they sell it to.
Moat:
Sources of a moat for Polynovo include:
- Regulatory approval - Polynovo have approval for Novosorb in North America, India, UK and Europe, Australia and parts of S.E Asia. This is a significant hurdle for new competitors to get over
- Hospital approvals - individual hospitals will assess and approve new products. Polynovo has a good history of expanding the number of hospitals is sells into.
- Becoming the standard of care. Once you become the go-to product it makes it harder for new players to compete. They will have to work hard and spend a lot of money on sales people to convince doctors to switch to their products. The more indications the product is useful for the greater the entrenchment.
- Lower cost - while I have not seen the pricing exactly myself, my understanding is the synthetic product is cheaper to produce than the equivalent biological product
The first 3 are kinda a tiered network-effect moat - First you need Country/Regional approval, then the hospitals themselves, then finally the doctors. In the end it becomes a network effect as you become the standard.
2 and 3 are still expanding for Polynovo with Novosorb as it adds more hospitals and doctors as its customers
Their Competitors
There are a lot of companies in the space, however not all of them are direct competitors.
Some examples are:
Integra Life Sciences
Integra produces a range of biologic dermal products used for wound reconstruction - some of these compete directly with Novosorb. Based on the last few earnings calls it seems revenues for these products are declining. With their guidance anticipating flat growth for these products for 2024.
Note Integra make the bulk of their revenue from products unrelated to Polynovo (e.g. Neurosurgery products and instruments)
Mallinckrodt Pharmaceuticals
Mallinckrodt acquired the company developing StrataGraft, a direct competitor to Novosorb, however according to their website the product is still in clinical trials.
Their Scalability
While Novosorb is a manufactured product, according to David Williams the facilities are not that capitally intensive. (It is a to-do to assess the capital cost of their factories). Worth noting they do have software like gross margins (~80%).
Existing manufacturing + the new facility (due to come online in 2025) should provide the capacity to deliver ~18x the devices manufactured in FY23.
Creating extra manufacturing, if required, should not be an issue and will be possible from the amount of cash they currently have + future cashflow.
The TAM is large, but hard for me to quantify in $.
As a guide, currently there are 145,000+ burns and soft tissues wounds in the US each year. I think an increase in sales of 2 orders of magnitude would not be unreasonable.
Management
Mr David Williams - Chairman
Experienced director and investment banker. Has special interest in the medical and pharma industry. Most importantly, used his own money to buy shares in Polynovo.
Stake: 21m shares (3.1% of PNV)
Mr Swami Raote
Was previously divisional head at Johnson & Johnson. Has no stake in the company as far as I can tell
A number of their senior leadership and board have stakes in Polynovo - while not large percentage-wise these do amount to reasonable amount in dollar terms.
Jan Gielen - CFO: 930k (0.14%)
David McQuillan - CTO: 670k (0.1%)
Leon Hoare - Director: 1.2m (0.18%)
Bruce Rathie - Director: 3.2m (0.47%)
Risks
They have a product which is (or at least seems) significantly better than the status quo and is being increasingly adopted, both in terms of their target market but also new markets (use cases). They have reached cashflow breakeven, have ~$40m in cash and basically no debt. From here I think there are 2 main risks:
- Execution - They have to continue to gain sales and produce quality product. It is critical that they maintain contaminant free and uniform product as they scale production. A single severe incident of contamination could cost them years of sales traction (if not the whole business).
- Disruption - A better product could come to market, perhaps that works in a in drastically different way. The benefit of their moat is it would take time for it to gain traction, but would not stop a better product with a good sales team (who have enough money).
I was able to find 2 products that could potentially compete with Novosorb both are apparently in trial stage:
- StrataGraft - Mallinckrodt
- DuraSorb - Integra
However I'd note they seem very similar (synthetic absorbable matrices) so not sure they would be sufficiently better to warrant adopting. However, with such great gross margins on offer, they could potentially compete on price particularly in the more price conscious markets.
Thesis
The thesis here is pretty straight forward - Polynovo to continue to increase market penetration and (therefore) revenue of it's Novosorb products.
Why I'd Sell
- A prolonged period (12+ months) of sales stagnation (that is, even if there no competitor, but suggests a saturated market or a failure of new sales execution)
- A new market competitor that takes significant sales from Polynovo's existing customers - even if Polynovo has continued top line growth
- A major incident involving the quality of it product.
- An acquisition buying spree - if Polynovo decides to use it's cashflow and buy other companies. A couple of good, strategic sounding, acquisitions is fine, but I'm not interested in owning a "roll-up".
FWIW here is RASK media looking at PNV
16-May-2024: Not exactly furious agreement between the brokers on PNV:



Yeah, that's a wide range, Target Prices from $1/share up to $2.75/share and 4 different calls from the 4 brokers - Sell, Hold, Add and Outperform.
Mind you, all four of them wrote those most recent client notes BEFORE this PNV Presentation at the Macquarie Australia Conference last week (on May 8th): PNV-Macquarie-Australia-Conference---Presentation.PDF
This announcement was released on the same day: First-$A9M-sales-month-and-$A10M-revenue-month.PDF
It may not be enough to shift those bearish brokers' views - or their target prices - but it looks positive to me:

I think they've passed an inflection point now, with positive cashflow and positive NPAT for H1, and about to announce results in August for a profitable full financial year (FY2024). The brokers arguments seem to be mostly that people are extrapolating too much sales growth occuring too quickly in the future and that the ramp up is likely to take longer than these bullish investors are expecting - and that PNV don't have the market all to themselves, so there's a competition argument from OM suggesting the R&D spend from PNV will need to remain significant if they are to stay competitive long-term. Well, Duhh! That's what they do.
I added PNV to my SMSF yesterday (Wednesday 15th May 2024) @ $2.17/share, and doubled my PNV position here also. It's not within my cicle of competence, however I'm confident enough that they have a viable product that works and is providing significant benefits, they are now profitable, they're growing at a decent clip, they have a long and wide runway of growth across the entire world and they've only really scratched the surface so far.
I think their results announcement and full year report in August might give the share price a boost, now that they're profitable and the growth across all important metrics is continuing at pace, so I don't claim to know much about the sector, but I reckon this one is de-risked enough for me to hold without a full understanding of every facet of the industry. I also hold CSL and I'm no expert on what they do either, but I don't need to be - coz CSL have their track record of outstanding TSRs over time - mostly through share price appreciation.
I don't mind David Williams - I had a good look into a few of the things he's done in the past last night - and posted a straw here on that - titled #Management - under PNV - and while I generally do not like management to be over-promotional, he's the Chairman, not the CEO or the MD - so he can hype the company up all he wants - and let the management get on with running the company. David is entitled to his opinion - he might not hold 5% of the company, but he holds enough, about $46m dollars worth (21.4m shares).
In terms of valuation, I wouldn't expect a company like this to look cheap, but they do look reasonably priced here considering their exceptional future growth prospects, and that's OK with me.
In terms of the wide range of broker target prices and calls, it suggests to me that either (a) they're not all "experts" in this field, or (b) experts when all given the same data can come to seriously different conclusions about what it is all likely to mean in the future. So what hope do I have? So ignore the valuations and back the management and the product - that's what I've chosen to do now. I haven't thrown the farm at it, but I've got exposure. PNV and CSL. My healthcare sector exposure. Plus some through Wesfarmers' new health division. Plus a little bit of NEU and PME here on SM but none in real life any longer. That'll do. Not in my wheelhouse (circle of competence), but I can live with that exposure to the sector.
16-May-2024. Not a valuation. A Price Target.
$2.60 was the average of member valuation estimates yesterday, and I'm adding 10% to that because I'm choosing to be a bit more bullish than average. So that's $2.86.
I added them to my SMSF at $2.17 yesterday and they closed at $2.16.
Price Target of $2.86 within 2 years and 4 months, so by September 2026.
Well run company producing quality products that are needed and add a lot of value, solving needs, improving outcomes for patients, now profitable, cashflow positive, growing all important metrics at a good rate.
I believe that becoming profitable is an important inflection point and that PNV's full year report in August is going to underline that to the market, and they just might get a positive re-rate off that.
I can easily look past their flamboyant and outspoken Chairman, who probably does more good than harm to be honest, so I don't have any issues with DW, and what I see, bearing in mind that this company is well outside my circle of competence, is a company that has a lot going for it; it's substantially de-risked - the risks are really only around the rate of growth, and the levels of profitability they can achieve - and over what timeframe.
I think it highly unlikely that some other company is going to invent alternative products that are going to be far superior to PolyNovo's and take away their market - I mean that COULD happen, but it could not happen overnight. Things don't move super-fast in health care - that much I do know. Adoption of new products and procedures takes time, and PNV are getting that traction now - they've been in the market long enough that they are being accepted and their products are being used - and that starts slow but does pick up speed, like a snowball, but it starts slow, and that's a competitive advantage that PNV already have over any new entrant to the market. So - while I don't know much, I think I'll be able to tell if PNV start losing market share to a competitor (or two) in future years.
'Nuff said. My Investment Thesis is neatly summarised in this document: PNV-Macquarie-Australia-Conference---Presentation.PDF [08-May-2024]
15-May-2024: Actually this straw is specifically about the Chairman, rather than about the rest of PNV Management.

Quite a character, our Mr. David John Williams. Chairman of three ASX-listed companies (PNV, IIQ, RMY) and past Chairman of at least three more companies - Medical Developments International (MVP.asx, for 13 years to 28-April-2023), Tassal Group (acquired by Canadian aquaculture company Cooke Inc. in August 2022), and Austin Group (family owned private company); David has also been a director of Select Harvests (SHV, 5 years to 18-Feb-2004) and Amcal which was acquired by Sigma Pharmaceuticals, now Sigma Healthcare, SIG.asx, who entered the ASX200 Index last week (on May 10th) when Boral (BLD) was removed. SIG's m/cap was recently significantly increased by their merger with Chemist Warehouse Group (a.k.a. CW Group).
David is also a lover of either red wine or lots of sunlight, perhaps both, and proficient user of photoshop one suspects.


With a social media presence and a flair for promotion.
David Williams (@DavidJ_Williams) / X
David Williams | Hort Connections

David Williams (businessnews.com.au)


David Williams is also Managing Director of corporate advisory firm Kidder Williams:

David Williams
David has over 30 years’ experience providing mergers and acquisitions, capital raising and strategic advice. Prior to establishing Kidder Williams, David was the Managing Director of Challenger Corporate Finance, Head of the Melbourne corporate finance office of SG Hambros, Head of M&A at ANZ McCaughan, and Head of M&A at Arthur Andersen.
David holds an Honours and Master’s degree in Economics and conducted Ph.D. research on Cooperatives and their capital structures. He is a Fellow of the Australian Institute of Company Directors. David is Chairman of ASX-listed companies; PolyNovo Limited and RMA Group Limited. [Also Chairman of INOVIQ Limited (IIQ).]
Kidder Williams:
Kidder Williams - Kidder Williams
We are a leading adviser to the food, agriculture and beverages industries, and also have extensive experience in medical and digital technologies.
We are globally connected and source international capital for Australian companies.

Kidder Williams Limited provide Corporate Advisory and Investment Banking services to private and ASX-listed companies, including: Corporate Finance Strategy, Mergers & acquisitions, Divestments and demergers, Capital structure, Equity and debt raisings and IPO. We are a leading adviser to the food, agriculture and beverages industries, and have extensive experience in medical and digital technologies. We are globally connected and source international capital for Australian companies. Kidder Williams Limited was originally part of the Mariner Group and set up on its own in 2005.
--- ends ---
OK, so, to recap:
David Williams is:
- the Chairman of PolyNovo Ltd (PNV), and owns 21.4m PNV shares worth around 3% of the company with a current market value of about $46m (@ $2.16/share);
- the Managing Director of corporate advisory firm Kidder Williams;
- the Chairman of INOVIQ Ltd (IIQ), and owns 5.43% of that company, that holding currently being worth around $2.7m;
- the Chairman of RMA Global Ltd (RMY), and owns 184.4m RMY shares, or 33% of the company, that holding currently worth around $12.7m;
- a previous Chairman of Medical Developments International Ltd (MVP), and a current substantial shareholder of MVP with a 13.35% stake worth around $4m (MVP's entire m/cap is now down to only around $36m); and
- a previous Chairman and substantial shareholder of Tassal Group Ltd, buying it out of receivership and arranging the sale of Tassal in August 2022 to Canadian aquaculture company Cooke Aquaculture Inc.

"It isn’t Melbourne investment banker David Williams’ first foray into the salmon game." Photo: Jesse Marlow
Source: Former Tassal Group owner David Williams back for second helping (afr.com) (22-June-2022)
David Williams has also previously been the Chairman of private family-owned company Austin Group and a previous director of Select Harvests (SHV, 5 years to 18-Feb-2004) and also of Amcal (now part of Sigma Healthcare, SIG.asx). Through his work at Kidder Williams, David has worked on numerous mergers and IPOs (SPC & Ardmona, Incitec Pivot), acquisitions (Bega buying back Vegemite), divestments and asset sales (SPC for Coca-Cola Amatil, Tasman Group to JBS S.A. of Brazil, selling Tassal out of receivership to Cooke Aquaculture Inc. of Canada), and other corporate actions, strategic reviews, recapitalisations, etc.

The man has some solid form, and is a rather unique individual.


Further Reading:
Medical Developments International (ASX: MVP) Providing An Alternative To Opioid Addiction - A Rich Life (11-June-2021, by "Downunder Value")
RMA Global Limited (rma-global.com)
Home - Medical Developments International
PolyNovo:
Revolutionary Synthetic Skin Substitutes | PolyNovo
PolyNovo | Healing. Redefined.






Some interesting information in the Macquarie conference presentation.
Two items caught my eye:
- A picture of the capacity resulting from the investment in new facilities. (Not see before although elements of the information have been disclosed before).
I don't recall them before ever having said that existing facilities support a capacity of 180,000 devices (please correct me if I am wrong).
The linkage to revenue is a bit ambiguous, but on one reading it does indicate that in FY25 existing facilities will max out if revenue growth is significantly greater than consensus c. 30% - which I expect it to be. It would have been good to hear the Q&A in the meeting!

2. It's a while since we've seen references to new products - and this time with an indicative timeline. Frankly, I am surprised to see the short term timeframe for SynTrel and Syntrix. But then again, we know surgeons are already using MTX internally, so perhaps its not such a leap.
I wonder if this means they've cracked the polymer property issues which appeared to be holding them back when this was discussed at the AGM. Must be - otherwise the short term timeline is foolhardy, to say the least.
It will be interesting to hear the commenary around this slide at the FY results.

There is a thread on HotCopper for PNV which is headed “the clinician’s speak”. There are an increasing number of positive studies and articles by clinicians about Novosorb BTM in the UK, US and India.
https://hotcopper.com.au/threads/the-clinicians-speak.6460688/page-15
There was also some excitement that the Newcastle upon Tyne hospitals NHS foundation trust bought £200,000 of BTM recently with a fairly enthusiastic ”legal justification”.
https://bidstats.uk/tenders/2024/W17/821166385
The collection of positive feedback accumulated by those who follow the company suggests that sales should continue to take off.
It also seems to be a product that can be mass produced at scale. My only niggle is there was mention in a recent co announcement and in DW’s podcast of a new distribution centre in Melbourne, and I do not know whether growth can be funded from cash generated and held.
Disc: held, dyor
On today's call, DW is referring to a comparison study putting Novosorb head-to-head with Integra's animal-derived product. I think this might be it.
It looks like a big deal - particularly given the overall healthcare economics findings, which is going to be important to the payors, particularly the health funds in the US. However, healtcare economics may also influence guidance in nationalised systems like UK, and reimbursement systems like Medicare in Australia.
==============================
Comparative Analysis of Animal-Derived vs Fully Synthetic Acellular Dermal Matrices in Reconstructive Surgery An Examination of Clinical, Aesthetic, and Economic Measures Timothy Olsen, MBA, Safi Ali-Khan, MD, and Derek Bell, MD, Annals of Plastic Surgery, Volume 92, Supplement 2, April 2024
Introduction: The fully synthetic skin substitute, NovoSorb Biodegradable Temporizing Matrix (BTM), may be a cost-effective alternative to the animal-derived Integra Dermal Regeneration Template (IDRT). However, the current literature insufficiently compares the two. Therefore, our study compared clinical, aesthetic, and economic outcomes in treating soft tissue wounds with IDRT, an animal-derived template, vs BTM, a fully synthetic template
Methods: Our single-center retrospective study compared outcomes of 26 patient cases treated with BTM (57.7%) or IDRT (42.3%) during 2011–2022.
Results: The mean surgery time was significantly shorter in BTM cases (1.632 ± 0.571 hours) compared with IDRT cases (5.282 ± 5.102 hours, P = 0.011). Median postoperative hospital stay was notably shorter for BTM placement than IDRT placement (0.95 vs 6.60 days, P = 0.003). The median postoperative follow-up length approached a shorter duration in the BTM group (P = 0.054); however, median follow-up visits were significantly lower in the BTM group compared with the IDRT group (5 vs 14, P = 0.012). The median duration for complete wound closure was shorter for BTM (46.96 vs 118.91 days, P = 0.011). Biodegradable Temporizing Matrix demonstrated a notably lower infection rate (0.0%) compared with IDRT (36.4%, P = 0.022). Integra Dermal Regeneration Template exhibited higher wound hypertrophy rates (81.8%) than BTM (26.7%, P = 0.015). Revisionary surgeries were significantly more frequent in the BTM group ( P < 0.001). Failed closure, defined as requiring one or more attempts, exhibited a significant difference, with a higher risk in the IDRT group (26.7%) compared with BTM (6.7%, P = 0.003). Biodegradable Temporizing Matrix showed a lower mean Vancouver Scar Scale adjusted fraction (0.279) compared with IDRT (0.639, P < 0.001). Biodegradable Temporizing Matrix incurred lower costs compared with IDRT but displayed a lower mean profit per square centimeter ($10.63 vs $22.53, P < 0.001).
Conclusion: Economically, although the net profit per square centimeter of dermal template may favor IDRT, the ancillary benefits associated with BTM in terms of reduced hospital stay, shorter surgery times, fewer follow-up visits, and lower revisionary surgery rates contribute substantially to overall cost-effectiveness. Biodegradable Temporizing Matrix use reflects more efficient resource use and potential cost savings, aligning with broader trends in healthcare emphasizing value-based and patient-centered care
Hopefully David Williams will expand more on the progress in India today in the meeting with @Strawmanat 11.00am. It looks very promising for the business with such a large population and a disproportionate number of burn victims. It will be a great outcome for burn victims also.
ASX Announcement - 4 March 2024
“The Indian Government has approved NovoSorb BTM to be included in the Government-e-Marketplace (GeM) portal.
GeM is a centralised procurement platform for Government hospitals that can now buy NovoSorb BTM throughout India. This approval provides access to supply BTM across all the Defence hospitals, Railways hospitals, ESIC (Employees State Insurance Corporation) hospitals and the various AIIMS (All India Institute of Medical Sciences) hospitals. We expect the first order within a month.
Simultaneously, our India team has been participating in several government tenders which will enable the Company to supply public hospitals.
Sales in private hospitals in India have been growing rapidly on a month-on-month basis.
PolyNovo participated at the National Academy of Burns of India Conference (NABICON) from 15 to 17 February 2024, the flagship event for burn surgeons. This was attended by 180 burn surgeons from all over India. In addition to a PolyNovo sponsored symposium, featuring U.K. surgeon Dr. Pratap Dutta, there were several other presentations featuring NovoSorb BTM by Indian and U.S. surgeons.
Chairman, David Williams said: “Our India team is optimistic we can win a number of other tenders that will open the doors of public hospitals for BTM.”
CEO, Swami Raote said: “It will be a huge step to be able to access burn patients admitted to public hospitals for treatment. NovoSorb BTM will enhance the standard of care and significantly improve the quality of life for Indian patients.”
Held IRL and SM
I made the mistake of IRL trimming PNV during the week but I did have a lot of them.
they seem to just be getting started, they are growing at pace, recently profitable and seem to be able to scale rapidly.
the reference in recent announcements to having to pay for a distribution centre over the next couple of years caused me to rethink the capex.
but the co has got to $4 a few years ago and is in a much better position now. I would expect this to fly there fairly soon. But it didn’t stop me from trimming.
I've already shared some analysis on the $PNV financial results in earlier Straws, which I do not repeat here. However, I commented earlier this week that David, Swami and Jan provided a lot of detail both in their voiceover on the presentation and also in the Q&A.
So, I thought I'd share the key nuggets I extracted from going back over the call and the transcript. In total, I think it provides a much richer picture of the strength of this business, and underscores my bullishness on it as a long term growth stock.
I'll bear all this in mind when I do my major valuation update at FY. There is a lot to consider.
So here goes.
--------------------------
My Overall Key Takeaway: Sustained, capital efficient, long-term growth, driven by existing and new markets, existing and new indications, and potential new platforms. $PNV is now profitable and cash generative.
Key messages
- Strategy and capital allocation - long term growth
- Financials - outperformed their internal budgets and plans
- Capex - material step up in FY25 to build "Mega" for $25m, but fully budgeted in 2022 capital raise
- Markets - now serving 37 markets with increasingly material RoW growing strongly
- Organisation - now built out, so future growth in headcount slowing
- Clinical development - clinicians leading broad expansion of indications; potential for multiple new platforms
1. Strategy and Capital Allocation
DW made clear the strategy is to keep expanding “both in indications and in geographies” wherever they can see the margins. He said this in clarifying feedback he has received on his statement that he doesn’t care about profits. The clarification is that by focusing on growth where he can see margins, then profits will follow. He tweaked his rhetoric by saying, “if you want dividends, you’ll need to sell some shares.”
2. Financials
All details covered in previous straws, but CFO Jan made the comment that they have achieved profitability earlier than budgeted because sales have been above plan
For example, the $8m month in November wasn’t budgeted until April
On cashflow, the business is essentially cashflow breakeven.
DW discussed reporting. He said there has been feedback on their approach of reporting "record sales months". He's discussed it with the Board, and they've decided to continue because they want to keep investors informed as key milestones are achieved.
3. Capex
On the new facilities, the main investment is yet to come
1H24 Capex of $1,1m was design work for facilities plus some R&D equipment
2H Capex “marginal increase in capex as the design process nears completion and we expect to commence construction in 1Q FY25.”
Total planned capex for the third “Mega” production line is $25m over two-year period
Guidance on the spend profile will be given once design is completed (FT24?)
Until new facilities are ready there are no issues with current manufacturing output form the existing two lines, which see continuous improvements in output and efficiency, evidence by the very low % Gross Margin.
Mega will be designed to be modular and scalable, as they plan to have to accommodate many more SKUs than at present.
(My note: Key risk to monitor: will procurement and construction costs increase materially since project first announced in end-22 when the design is complete and contracts let in FY25? A 25-50% cost blowout would not be unprecedented. While that would not be good, it is not really that material, overall.)
4. Revenue & Markets
RoW sales are becoming material "from 16% of total sales to 24%"
Now have sold product into 37 countries
Key market details (not all presented but covered in voice-over):
After not having raised prices in US for several years, there is now an agreed approach for price revisions
In the US, “narrowing the gap” to the market leader in the “difficult burns” category
BARDA trial now 91 patients enrolled; 1st patient enrolled in India (2 centres approved). Looking at options with FDA and BARDA. Base option is to get to 120. Enrolment expected to be complete by May. There will be announcement when it is decided how to close out the trial to a meaningful close. Also working with another FDA agency to see how “real world” data can be sed to give added claims into the trial.
Strong growth in ANZ where already #1 in burns is largely outside of burns
India – “half a dozen tenders” under way. “Getting good soundings.” “Very optimistic that in the very near term we’ll have something to say.”
HK also continues to “trade well”. China – pathway identified, but not yet going beyond HK. Developing plans for extending into Shenzen area (GBA).
Germany: market leader in “Advanced Dermal Substitutes” (4th largest market after US/UK/ANZ – note: Ger. is a distributor market)
Turkey – large initial sales
Middle East – sales driven by a physician who relocated to ME from East Coast US and wanted the product
Japan – have a partner defined and a lead KOL. Working to see if data already submitted to US FDA can be used for submission in Japan with MoH, KOLs and reimbursement agencies.
New demand arising from war zones in Ukraine ($1.2m sale in February - not 1H; “we believe another coming”) and Israel. They believe they are getting some sales orders from other countries that are ending up in these locations, as well as charities.
Several ongoing discussions with charities, UN agencies, Gates Foundation, WHO, MSF, etc. to help get the product to countries that otherwise can’t afford it.
5. Organisation
Headcount +64 on PCP, but only +19 in 1H FY24
Plan for 2H FY24 from 237 to 260 +(23-25)
- US sales: currently 75 (65 reps + 10 managers) going up to 85
- ANZ sales
- R&D and Manufacturing
- Back Office: now have the full teams and scalability
Completing the build-out of the senior management team
- HR lead has started in last few weeks
- Chief Medical Officer joining – leading on FDA and BARDA conversations
- General Counsel about to be appointed
- Close to appointing APAC lead, for entry to China and Japan, and other countries
6. Clinical Developments
My key takeaway: It is clear here is the potential for many years of development leading to new platforms and multiple groups of new indications. Most of this is clinican led.
A key observation is just how important the customer-led (clinicians) work is. (My Note: Just reflect on the following points and consider the tiny R&D budget. I don’t think I’ve ever seen this before in healthcare. There is so much upside to come.)
DFU study: stopped after 25 patients because not getting right wound debridement. Protocol to be re-written and brought back in-hospital (rather than outpatients) to get great consistency needed for a successful trial. Expecting to focus more on to limb salvage – new trial, Announcement on trials coming in a few weeks
Prof Marcus Wagstaff trained 30 surgeons in UK who then took the knowledge onwards to Ukraine
Much clinician-led development taking place into new indications; 230+ publications (214 at FY23), “literally across the entire clinical spectrum”
Customers proposing BTM could be a replacement for allografts (papers on this). Potential to upshift and replace grafts
Several authors proposing that BTM could be a good solution in low and middle income countries where other technologies are constrained
Overall global markets outside US and W. Europe and a few market in APAC “on the fringes” (hey! Swami, that’s no way to talk about ANZ) – we are focused on sustainable, global growth
MTX rolling out in US – demonstrating great outcomes in open abdominal and dehisced wounds. Will start compiling evidence to allow MTX to be rolled out globally. Focus has been using MTX with “expert clinicians” on complex applications, and expecting wider roll-out from July.
Working to be differentiated in connecting KOLs across the world who can teach other surgeons in how to treat acute complex wounds.
$PNV already recognised in burns and trauma, and now looking to go beyond these into:
- Oncological resections of head, neck, scalp, skin and oral cancer
- Potential to use in infections (necrotizing fasciitis and hidradentis) where competitor products cannot deliver
- Getting into complex vascular space to save limbs from being amputated
Developing an implantable platform in hernia and breast. Still not happy to share timelines, but happy with the feedback getting from clinicians. Addressing how to build a “sustainable platform in the implantable space”. (Sounds like still some time off, but I still think this is OK given the growth potential of BTM and MTX)
Working on developing Novosorb Mesh product, currently bench testing, testing with animals, and sharing it with clinicians to establish their expectations on added strength and flexibility. Work is being done to compare with the market leader.
Disc: Held in RL and SM
Three updates this morning - seems about right.
Superbull Macquarie slowly coming into line!
While I am not running my model update until FY, I'll tweak my val. up by 5% just so as not to be too out of whack with the new information.

Disc: Held in RL and SM
Dermal repair company $PNV reported its 1H FY24 results this morning. The major elements of 1H have been pre-released in the “Trading Result” announced over a month ago. This set out the sales results, which were very strong, as well as anchored the key financials. So, I expect that today will be less about the result itself and more about the trajectory towards the FY. More on all that below.
Their Highlights
The half year audited results attached to this release show:
- Record 1H FY24 sales of A$42.2m up 54.9% on STLY of A$27.3m.
- Total revenue including BARDA of A$48.8m up 65.6% on STLY of A$29.5m.
- Strong growth in U.S. achieving record sales of A$32.2m up 41.7% on STLY of A$22.8m.
- ROW sales of A$10.0m up by 122.2% on STLY of A$4.5m including strong performances in ANZ, UKI, and the Middle East, also growing sales in India, Hong Kong, and Canada.
- The Group recorded a net profit after tax of A$2.7m (1H23: A$3.8m loss).
During the Period, the Company’s other key initiatives and achievements include:
- Exceeded $8 million monthly NovoSorb BTM sales for the first time in November ($8,810,000).
- Appointed Chief Medical Officer and Chief People Officer.
- Additional funding of US$10 million from the Biomedical Advanced Research and Development Authority (‘BARDA’) for the pivotal trial of NovoSorb BTM in full thickness (third degree) burns.
- Passed the mid‑way point for the recruitment of the pivotal trial, with 90 patients currently enrolled.
- Increased sales teams and customer base globally, 861 hospital accounts and 237 staff.
- Progressed the product pipeline for NovoSorb BTM and NovoSorb MTX and developed surgical mesh prototypes for hernia repair.
- Finalised concept design and commenced detailed design of additional, new manufacturing facility in Port Melbourne.
Context for today’s result (you can skip this as it is a little self-indulgent!)
SP action for $PNV is a rollercoaster that sometimes defies belief, bearing little relationship to the business fundamentals. A bit like riding a rollercoaster in the dark, where you cannot see if the next move is a soaring climb, or a plummeting fall that risks bringing the contents from the last fast food stall you visited before the ride back up! Entertainment value is added by Ride Master David Williams – red-jacketed, red-faced, and gesticulating, wildly to “Roll-up. Roll-up” as he waves at you with a 30cm piece of BTM, while CEO Swami in the background cooly explains the genius of the engineering that allows the ride to function, assuring punters of their safety.
$PNV reached its most recent low point in October. This was driven by a mixed market reaction to the growth of the cost base supporting the global acceleration (clearly signalled at the 2022 capital raise), raising doubts as to the path to profitability, not helped by Ride Master DW saying he didn’t care about profits.
However, since that time, four factors have driven a sustained 5-month uptrend of +80%:
1) the release the $8m/($9m record month in December,
2) the January pre-released Trading Result with preliminary bottom line,
3) the record single sales order of $1.2m and
4) broader, macro-risk-on.
But this is a rollercoaster, and we are still some way off the lofty heights of $2.69 reached in the run-up to 1H FY23, so you never know how far the climb continues or whether we temporarily lurch downwards once more before recovering.
Through all this, I try not to let the “Buy, Buy”, “Sell, Sell” trader-analyst-fundies distract me. They’re not much help really with an average Target Price of $1.95 representing a wild range of $1.00 to $2.90 – materially down on 12 months ago ($2.53, $1.90 - $2.90).
My model is at $2.00 ($1.63-$3.30) with my eye clearly focused on the disproportionate upside potential, even though I have also come off my position in Sept-22 ($2.46, $1.62-$3.28).
So, with the scene set, what do I make of today’s result?
My Analysis
On the release David has said: “There is little new here that was not in our 22 January announcement. It was a great half, but we have moved on. There is a lot to talk about that has happened since 31 December, which we expect to talk about during our investor webcast on 27 February.”
In other words “Roll-up Roll-up to the David, Swami and Jan show at 1pm AEST today!
Important Note: My analysis below may differ to what is presented today. In fact, it will, That is because $PNV typically make various adjustments and report underlying numbers in their presentation, whereas I stick to the audited accounts. That said, no-one audits my analysis. So, all care, no responsibility!!
Revenue
There is nothing to add on sales to the detail I gave in my straw on 22-January. Revenue (which includes BARDA) is up 65.6% - a slight acceleration from the PCP. Sales are up 54.9%, with the US up 41.7% and RoW up 122.2%, with sales in several new markets.
With a FY revenue consensus of $101.4m, revenue in H2 needs to hit $52.6m, which would be growth over the pcp of 42.0%. So, how likely is this? Well, H2 growth rates in FY22 and FY23 have been 43% and 56%, respectively. And looking at the last three years, there is no clear 1H / 2H trend. H2 FY24 also has the boost of at least 1 large order to Ukraine valued at A$1.2m. And with the recent impetus in RoW from the expansion of the global sales and marketing footprint, everything points to a strong finish to the year. My model is for FY sales of $105m for the FY. But I am a $PNV bull, so DYOR!
Gross Margin
Gross Margin comes in at 95.9%, compared with 94.5% in pcp. Overall,it is in the usual ballpark of 90-96%. This is expected as the direct fixed costs of the current facilities are recovered over progressively increasing volumes. However, when the new facilities come onstream in FY25, I expect %GM will drop back sharply, as the new facilities have been sized to support sales up to $500m p.a!
Still, compared to the competition in dermal repair, $PNV has an extremely high %GM.
Opex
Opex (excl. D&A) grew 46% from $30.6m to $44.6m, a slower rate than 107% in the PCP. Importantly, it is now growing at a slower rate than revenue. Opex is now 91% of revenue, down from 104% in pcp. Yay!
This moderation was expected for two reasons, First, a major expansion of the workforce occurred during FY23, following the capital raise, to pursue the broader global sales strategy. While expansion has continued in 1H24, there has been a greater focus on execution. The second reason is that the FY23 comparison was distorted due to some items relating to former CEO compensation. In the presentation this is one of the “underlying” corrections that Jan has made.
That said, corporate costs have expanded significantly, given the senior hires indicated above. However, $PNV is not a truly global business, and you need functional heads capable of delviering their roles in that context.
Within Opex, R&D continues to expand. This is important and welcome, as without ongoing innovation $PNV can never become a long-term winner in dermal care. Management seem to be applying capital discipline here, holding R&D/Revenue at 10%, in order to deliver their commitment to getting to profitability.
So, overall, I am very happy with the progression of the Opex profile.
Profit and Cash
By my calculations, EBIT is $1.1m (up from -$3.8m) – an improvement of $4.9m.
NPAT is $2.7m, up from a PCP loss of -$3.8m – an improvement of $6.5m, assisted by the Tax refund of $1.6m.
So, well done David, Swami and Jan and team. You are on track to delivering your commitment at the FY22 Capital raise to be profitable in FY24.
Cashflows align quite well with the financials. We are now just Operating Cashflow positive, at $0.58m compared with -$2.73m in PCP. And by my measure of FCF (which includes all capex), they are close to breakeven at -$0.48m.
It is worth noting that capex has increased from almost nothing to $1.1m, as the build the new production facilities. This should be expected to ramp up significantly over the next 12 months. So, hopefully, management will guide on that in the presentation this afternoon.
The balance sheet is strong with Cash and Equivalents at $45.58m, and debt at $2.2m (current and long term) is negligible and being paid down.
My Key Takeaways
Solidy on track. Continuing strong revenue growth and management demonstrating cost discipline to meet their commitments. May it continue.
Valuation
I’ll update my valuation after the FY24 results. For now, I am content to stay at $2.00 ($1.63-$3.30)
However, I can say that if execution continues in this manner, I'll be upgrading as the downside cases in my model start to fall away.
Disc: Held in RL and SM (with high conviction)
DW as ever quick to make sure everyone on the mailing list gets the Macquarie update on $PNV following yesterday's trading update.
Macquarie are the most bullish of the bulls, and lifted their $2.70 Price Target to $2.90 on the trading update that came in 8% ahead of their revenue number.
Accordingly, their FY revenue forecast is $103.0m, which is about where I am.
The Macquarie price target is well ahead of my central view (update this morning to $2.12) as they are more aggressive on revenue growth and profitability in the early years. I can easily get to the Macquarie valuation, as my upper scenarios are north of $3.00, so they are in my view well within the realm of reason.
$PNV remains one of my favourite holdings. After a few years of trading sidesways - and down more often than not - 2024 could be a breakout year for the company as it moves to deliver its first NPAT - particularly if it can maintain >50% revenue growth, which appears likely as super strong ROW progress offers the prospect of offsetting any moderation of US growth that may occur over coming years.
Disc. Held in RL and SM
$PNV has issued its 1H trading update, which it usually does around this time.
Their Highlights
• Total global revenue including BARDA was A$48.8m, up 65.6% on STLY of A$29.5m.
• Record 1H24 sales of A$42.2m up 54.9% on STLY of A$27.3m.
• U.S. 1H24 sales of A$32.2m up 41.7% on STLY of A$22.8m.
• Rest of World sales of $A10.0m up 122.2% on STLY of A$4.5m including strong performances in ANZ, UKI, and the Middle East, also growing sales in India, Hong Kong, and Canada.
• BARDA revenue of $A4.9m up 133.1% on STLY of $A2.1m. Currently 83 patients enrolled in pivotal clinical trial.
• EBITDA, EBIT and NPAT all positive. EBITDA $1.9m, up $4.4m on STLY EBITDA ($2.5m)
• Underlying EBITDA $3.6m (excl. non-cash items)
My Analysis
With total revenue up 65.6% to pcp, we are now seeing the expected acceleration. (The last two pcp comparison for 6m reports have been +56.3% (2H23) and +62.1% (1H23)), which means we are seeing the benefits come through of 1) the early 2023 expansion of the US salesforce and 2) the entry intro new territories.)
Digging into the numbers, this growth was only possible due to the expanding BARDA trial, without which we'd have seen growth of 55% (still decent), driven by US with 42% (Recall US FY23 growth in cc was 34.0% in CC, so it will be interesting to see the cc number when the full report comes out. However, 42% should be good as it comes at a time of greater fx stability and potentially even a stronger $A in Q4).
ROW ticking along nicely too at 122% off a small but increasingly material base.
It is also good to see the report for "EBITDA, EBIT and NPAT all positive". The NPAT bit is the key commitment made at the last capital raising, which has been re-affirmed at each report, even though DW claims not to be concerned about.
Overall, provided $PNV maintain a reasonable cost discipline then the second half could lock in a decent maiden profit.
$PNV appears on track to my forecast of $102m for the FY.
Overall, on track, and in line with expectations.
Disc: Held in RL and SM
Nice to see director buying Dr Elliott doubling her holding on market trades (38k purchase for context)
Information or documents not available now must be given to ASX as soon as available. Information and documents given to ASX become ASX’s property and may be made public.
Name of entity PolyNovo Limited ABN 96 083 866 862
Introduced 30/09/01 Amended 01/01/11
Appendix 3Y Change of Director’s Interest Notice
Appendix 3Y
Change of Director’s Interest Notice
Rule 3.19A.2
We (the entity) give ASX the following information under listing rule 3.19A.2 and as agent for the director for the purposes of section 205G of the Corporations Act.
Name of Director Dr Robyn Elliott Date of last notice 30 June 2023
Part 1 - Change of director’s relevant interests in securities
Direct N/A
31 October 2023
In the case of a trust, this includes interests in the trust made available by the responsible entity of the trust
Note: In the case of a company, interests which come within paragraph (i) of the definition of “notifiable interest of a director” should be
disclosed in this part.
Direct or indirect interest
No. of securities held prior to change
Class
Number acquired by Dr Elliot Number disposed
42,789 ordinary fully paid shares
Nature of indirect interest
Note: Provide details of the circumstances giving rise to the relevant interest.
(including registered holder)
Date of change
Ordinary fully paid shares.
32,000 ordinary fully paid shares.
Nil.
Note: If consideration is non-cash, provide details and estimated valuation
No. of securities held after change
$38,080.00 paid by Dr Elliott to acquire 32,000 shares at $1.19 per share via on market trade.
Value/Consideration
Across all holdings:
74,789 fully paid ordinary shares.
+ See chapter 19 for defined terms.
01/01/2011
Appendix 3Y Page 1
ME_206737525_4
Appendix 3Y
Change of Director’s Interest Notice
Shares acquired via on-market trade.
Example: on-market trade, off-market trade, exercise of options, issue of securities under dividend reinvestment plan, participation in buy- back
Nature of change
Part 2 – Change of director’s interests in contracts
Note: In the case of a company, interests which come within paragraph (ii) of the definition of “notifiable interest of a director” should be disclosed in this part.
Detail of contract N/A Nature of interest N/A
Name of registered holder N/A (if issued securities)
Date of change N/A N/A
No. and class of securities to which
Note: Details are only required for a contract in relation to which the interest has changed
interest related prior to change
Interest acquired N/A
Interest disposed N/A
Value/Consideration N/A Note: If consideration is non-cash, provide details
and an estimated valuation
Interest after change N/A Part 3 – +Closed period
Were the interests in the securities or contracts detailed No above traded during a +closed period where prior written clearance was required?
If so, was prior written clearance provided to allow the N/A trade to proceed during this period?
If prior written clearance was provided, on what date was N/A this provided?
+ See chapter 19 for defined terms.
Appendix 3Y Page 2
01/01/2011
ME_206737525_4
October 23
Given Polynovo was a popular topic of conversation yesterday at the Brisbane meetup, I thought I might share my valuation methodology for some feedback after updating for FY23 numbers.
My valuation is based the following assumptions:
- Cost per employee and other expenses as a percentage of revenue are relatively stable.
- Gross margin of 85%. Is this correct/conservative?
- I am conservatively (or maybe not??) assuming the growth isn't going to continue or accelerate from current position.
- R+D of $5-6mil a year.
- Assuming a PE40 in FY28 with 15% discount rate (required return). In all cases this gives a PEG of less than 2.
- Additional 70 employees a year to create the growth.
The two major factors contributing to the profitability of Polynovo and hence my valuation after the above assumptions are:
- The increase in revenue over time
- The extra cost of the additional employees which enables the revenue growth.
I'll start with where I get my numbers from. The table below extracts the % in comparison to revenues from previous years to be able to make the assumptions going forward:

My base, stretch and bear cases are below, orange cells are inputs with the highlighted yellow being the discounted back valuation. My final valuation is the average of the three at $2.19.



Would be extremely interested in any feedback and thoughts on the above! Models are never correct and my model above is somewhat simplistic. When I do the numbers in the way I have Polynovo looks like a potential cash printer but I don't know if my numbers further down the line are a bit too optimistic!
Morningstar equity research initiates coverage on Polynovo with a 2 Star (**) (slightly overvalued) rating
There's more detail in the full analyst report but FWIW the summary snippet below captures the general gist
Polynovo (ASX: PNV)
We initiate coverage on medical device provider Polynovo with a fair value estimate of $1.00. Shares are currently overvalued, trading at a 34% premium. Morningstar equity analyst Shane Ponraj suspects the market is likely too optimistic on the speed and extent of Polynovo’s commercial rollout and is underestimating competitive pressures
Ponraj thinks the market is also overly excited about potential new indications of Polynovo’s NovoSorb technology. While broader indications including hernia repair and breast augmentation and reconstruction are being considered and would expand Polynovo’s addressable market, these are still very early in the development phase. Our Uncertainty Rating for Polynovo is Very High, and we assign a Standard Capital Allocation rating
Polynovo’s main product, NovoSorb BTM, is a patented biodegradable synthetic scaffold to support the regeneration of the dermis when lost through surgery, trauma, burns, or other causes of tissue loss. Polynovo’s current strategy revolves around increasing its sales staff, expanding its geographical footprint, and exploring new uses for its NovoSorb technology beyond the dermal substitute market. With its geographical reach, the firm estimates its products are available to 800 million people as of fiscal 2023, but highlights that the global market is underserved
Ponraj does not award Polynovo an economic moat given low switching costs for clinicians to adopt competing products and concerns over the durability of intangibles related to NovoSorb. He thinks Polynovo will have little to defend its position when faced with stronger competition in the coming decade, particularly when its key patents expire in fiscal 2028
Financial success in medical devices is also dependent on distribution networks, hospital relationships, brands, and marketing expertise that larger competitors may already have and can utilise more effectively
Why I Wish He Wouldn't
I couldn't help myself write this straw on why I wish David Williams would change his investor relations behaviour. The commentators I have seen since yesterday's trading update announcement have made three statements about yesterday's release:
- Why report two months sales data compared to pcp, when the quarter end is a few days away? (There wasn't even an $8m month to at least be consistent with previous patterns of reporting) Particularly when there isn't really anthing to disclose.
- The growth results are strong, fair enough. (Particularly given the languishing SP)
- It doesn't change views on valuation
That's pretty much where I was yesterday in my straw.
But, you say, the market has moved up 14% in the two days. True, but the stock is still down 50% from its current 12m high in February.
So here is why I don't like David's "cherry picking reporting" and it needs a graph to explain. Note that the numbers below are made up, as they seek to illustrate a general point that is applicable to $PNV. The calculated rates of growth are relevant, however.

So here's what it shows. (Don't worry about the absolute numbers, I just picked 100 as an arbitrary starting point!)
Blue Line (acutally a curve), starting at June-22 at 100, this line grows each month at a compound monthly rate of 3.99% or 60% p.a. - which is in the ballpark of $PNVs current annual sales growth.
Orange line: Jul-22 and Aug-22 are depressed below the trend by 20% - indicative of the lumpiness we know exists month to month in $PNV sales. Let's assume we had two bad months at the beginning and two strong months at the end. The "lost" sales from the beginnining are added back in above trend in Jul-23 and Aug-23. So the total sales over the 14 month period is the same for the Blue curve and the Orange curve. It's just that the blue curve is perfectly smooth and on trend while the orange curve is lumpy at each end.
This is my suspicion of what could be going on. Two soft months cherry-picked at the start and cycling two strong months at the end for pcp comparison.
Red Line: So, let's do the maths. What's the ANNUAL SALES GROWTH rate from Aug-22 to Aug-23 (or Jul-22 to Jul-23)?
Blue Line = 60% growth. Red line (for orange curve) = +125%. Over double the underlying growth rate.
That's how misleading annual growth numbers can be if you cherry pick your data-set.
Which is why the high reported numbers in yesterday's ASX release from a management team with a reputation for cherry-picking data shouldn't necessarily result in any change in our view of the underlying value of the business.
Of course, the good news is that, even if the release represents "peak cherry picking", its does mean that underlying revenue growth is still in the region of 50-70%, whereas the market is expecting +42% this year and +34% in FY25.
As a result, no analyst upgrades should be expected, apart from the bears who have a SP <$1.50 or so. (There is one in my dataset with a price target of $1.08. Maybe they'll wake up one day).
Life would be much easier if we got any of the following:
a) Monthly sales, every month or
b) Quarterly sales, every quarter or
c) HY and FY results. Period.
Of course, I could be wrong, and perhaps the underlying trend is stronger. I hope it is. But that's the problem with management who are too promotional and inconsistent in how they report. You just can't tell.
If I wasn't so high conviction on the product and the business, I think I would have given up long ago due to exhaustion.
No doubt, the David Williams Circus will continue to roll on. So,... roll up, roll up.
Disc: Held
Polynovo has just requested a trading halt for the following reasons:
• The trading halt is requested in respect of a trading update and upgrade
Unless otherwise requested by the Company, PNV requests the trading halt to remain in place until the earlier of the release of an announcement or Tuesday 26 September 2023.
That’s a much needed announcement for the share price! What’s DW got in store for us this time? Perhaps it’s another new monthly sales record?
David has kindly emailed around the recording of $PNV's presentation at the Bell Potter Emerging Leaders Conference 2023. Here it is for those not on the mailing list.
https://youtu.be/scQoF-zaZrY?feature=shared
Many are stories we've already heard (some again and again ... and again).
But there are a few nuggets of interest
India
DW says they are now generating sales in India. In a separate statement he says they want to hold consistent pricing internationally. "Even if we made the decision to go into India at half the price, that we're charging in the US or Australia, we'd still be making a margin in excess of 80%."
In my recent valuation, I have been more bearish on emerging market pricing, running scenarios at 25%, 30% and 35% at modest volumes.
I couldn't imagine that DW would still be using a 50% benchmark (implying its a lower bound) unless they were generating sales in that ball park. Its difficult to avoid reading the tea leaves, and I probably just have to be patient until Feb/Mar 24.
Germany & Europe
In providing some further details about Germany, David confirms Novosorb is the standard of care there. This was also shown on a recent presentation. He notes that although their preference is to market directly, they are using a distributor in Germany because the distributor already has a complementary product. Perhaps this goes some way to explaining FY23's +189% German sales result.
DW noted that they are now also selling in France and Spain.
USA
He said that the product is getting more traction with diabetic foot ulcer and venous leg ulcers in the USA. He said they are considering hiring a specialist sales force for the podiatric surgeons OR are considering a specialist distribution deal. Again, Swami spoke about this pretty much on day 1, and a year later they are still mulling this one over.
Focus & Blue Sky
The other key message confirms that they are focusing on broadening the clinical applications of the current products, or rather, staying close to the surgeons who are leading the innovation.
David finishes by flashing the "blue sky" applications. We spent a lot more money on R&D in the last two years,... we used to have a man and his dog ... now we must have about 8). "We've got people developing new products for us",...with an update on the second patient now treated with the diabete drug elution product at Royal Adelaide Hospital.
Of course, the tangible measure is when DW announces the next milestone month, which would be $9m.
In summary, there is nothing really new here, but I like listening to understand how the messages and emphasis are evolving.
Disc: Held in RL and SM
I know a few other StrawPeople follow $PNV closely. So this is a question of detail for them.
I've been trying to get my head around the statement: "U.S. NovoSorb sales $46.1m up 44.6% (34.0% in local USD)"
We don't get consistent reporting of numbers, so I have constructured the following table for US Sales in A$ and US$. (Warning - shaded numbers are my calculations and have not been reported by $PNV and may be wrong, particularly given that I am using an annual average FX when volumes vary significantly through the year).

The numbers I am focused on is the constant currency USD sales, %y-o-y difference.
My estimates of the FY-end US Sales Force (FTE) are as follows (note: not reported)
2021 = 36 (reported)
2022 = "54" (At end 2021, DW said Ed would add another 20,... I assume he came up a couple short)
2023 = "75" (And then the same again in FY23)
Note - the last two numbers may be inaccurate, but as far as I can tell, they haven't disclosed US sales force numbers for a bit. DW has thrown some numbers around, and there is as far as I can tell now a sizeable non-Sales US headcount. However, directionally, the numbers make sense given 218 total reported at 30-June-23.
Forget, the fine detail, but doesn't this point to a significantly slowing of US$ sales/ salesperson? We know it takes a year or two for new starters to ramp up their sales volumes. So, given the big increase in numbers over the years, shouldn't there be a sizeable lag effect as productivity of sales staff added 2 years ago continues to grow. So, however you cut it, incremental USD sales of $7-8m FY22 to FY23 appears light.
Maybe something structural is happening. Maybe the initial workforce covered high-use burn clinics, and now incremental workforce are hitting lower returns, with new accounts being less valuable in general hospitals. (By the way, that is an entirely rationale sales and marketing strategy.)
IF (and it is a big if) this is real, its going to masked in FY24 by big numbers coming form emerging markets, and we won't find out until some time down the track that saleforce productivity or market penetration is flattening off.
I feel like I am having to play Hercule Poirot here, and just wish they were more consistent in their reporting. But before I fire off a missive to DW, has anyone else sensed anything?
Has anyone else had a look at this?
$PNV reported their FY23 results, and before heading into the David and Swami show, I am reporting my quick take.
Top line: strong revenue growth slightly ahead of market expectations at +59% (alebit behind my model), but with a significant expansion in costs, with total expenses up +66% largely driven by expanding the sales force into new markets. This drove an increased net loss of A$4.93m (FY22 A$1.19m), which they go on to say is essentially flat after adjustments (departed executives ... the gift that keeps on giving).
Their Highlights
- Total revenue including BARDA of A$66.5m up 58.8% on prior year of A$41.9m
- Strong growth in U.S. achieving sales of A$46.1m up 44.6% on prior year of A$31.9m
- ROW sales of A$13.5m up by 133.9% on prior year A$5.8m
- The Group recorded a net loss after tax of A$4.93m (2022: $1.19m loss).
- The loss in the prior year FY22 included the reversal of A$4.7m in share-based payments from the forfeiture by the previous CEO and COO on their resignations. The underlying net loss after tax excluding non-cash items is A$2.32m (2022: $2.00m loss). At year end the business had A$46.8m cash on hand.
- Excellent sales growth can be explained by the genius technology that is NovoSorb BTM and NovoSorb MTX, and by an increased sales force and geographical expansion. However unprompted surgeon engagement around the world in trialing the product, and publishing and presenting their results and developing new uses for the product has been amazing.
The Company’s other key initiatives and achievements include:
- First $7 million sales month in May 2023 (May 2022: $3.3 million), Total Revenue $8.3m in May 2023
- $53m capital raising
- 510(k) clearance from the FDA for NovoSorb MTX and saw first sales to plastics and reconstruction
- Entered Hong Kong, India, and Canada markets and saw early sales
- Grew the U.S. team from 54 to 93 and increased U.S. customer accounts from 189 to 299 hospitals
- In our direct markets including the U.S., we increased our customer accounts from 470 to 638
- Increased staff worldwide from 152 to 218
- Enrolled 64 patients into the U.S. BARDA pivotal burns study (53%)
- Enrolled 25 patients into the U.S. DFU Chronic Wound study for health insurance reimbursement (18%)
- Enrolled 35 patients into the chronic wound study with Flinders University South Australia (55%)
- Leased an adjacent property in Port Melbourne to significantly increase manufacturing capacity
- Awarded Victorian Government grant for manufacturing Diabetic Foot Ulcer product (NovoSorb SynPath)
My Analysis
With a strong cash position post the capital raising late last year, $PNV are making good on their investment in sales and marking to accelerate sales.
So, the 43% expansion in headcount (surely, mostly in sales and marketing) is hardly unexpected, and is their fastest expansion for a while. And given that we know it takes up to a year for a new sales and marketing employee to break even, such a scale of investment comes at a necessary cost.
Cash is still being burned. A total of -$8.3m (including leases) which is up from -$2.4m in FY22 (where I am excluding the benfit of the sale of the Melbourne property). With $47m in the bank, the rate of burn is not a concern, although the major spend in the new manufacturing facilities is still to ramp up – so we should expect to see significantly more capital investment in FY24.
Global hospital accounts at 638 are up 35%, and of course new accounts are a leading indicator of the sales growth to follow.
In both the report and the presentation, prominence if being given to the number and breadth of publications demonstrating the expanding clinical applications physicians are finding. And those of us on the DW mailing list have seen the benefits being report. So, another leading indicator of future growth.
The presentation is light on detail in terms of market progress. We can see that US Novosorb sales grew 44.6% (although only 34.0% in USD) and RoW sales reached $13.4m up 133.9%.
On sales, the US constant currency rate is slower that I expected, so it will interesting to hear how this is characterised on the call this afternoon. (There is no evidence of a boost from Integra’s recall of Surgimend.) With US accounts expanding by 58% to 299 from 189, and existing accounts expected to grow sales, the US sales growth number is a bit soft in my view.
RoW is starting to become more material, and this is a key number to watch in the future. Several of these markets including Canada, Hong Kong and India only started during FY23, with several only really getting going in H2. Of course, this is where a lot of the sales and marketing expansion has been, so it is not surprising.
I’ll leave it at that ahead of the call.
My Key Takeaways
Overall, there is something in the result for every thesis. Bears more focused on the short term will point to increased cash burn, rising costs and slowing US Sales. The bulls will point to expanding market footprint with strong RoW becoming material, new accounts, top line growth, and growing positive clinical evidence.
I remain in the bull camp based on this report. But I can see a stronger focus on revenue growth pushing the cash generation profile backwards, and so there is nothing in this report for me to upgrade my valuation. If anything, the upper part of my range is scaling back. But that’s for later in the year.
Based on this result, I am a solid hold. But I want to learn more about US progress, as I am sure others on the call will too.
Disc: Held in RL (4.3%) and SM
Latest email from DW. (I chuckled at his remark about the "Britishness" of the article. Those of us who have experienced David's flamboyant approach to communication will get the joke. But I think that is also a difference between investor relations and publishing in a serious peer-reviewed journal.)
Key Take-away: Growing evidence supporting breadth of potential in BTM in complex wound care.
Side question to StrawMedics: Does anyone know why BTM isn't FDA-approved for full thickness burns, whereas it is approved for this indication in so many other jurisdictions?
----------------------------------------------------
EMAIL from DW follows
Kidd T, Kolaityte V, Bajaj K, Wallace D, Izadi D, Bechar J.
The use of NovoSorb biodegradable temporising matrix in wound management: a literature review and case series.
Journal of Wound Care. 2023; 32(8):470–78.
https://doi.org/10.12968/jowc.2023.32.8.470
This is a retrospective observational case series evaluating the use of NovoSorb BTM in 37 patients across a wide range of wound types including acute trauma, hard-to-heal (chronic) wounds, acute infections, and skin cancer excision.
Data reviewed included patient demographics, wound characteristics, time to BTM integration, time to skin grafting, and the incidence of complications.
Successful outcomes were achieved in most cases, despite the type of wound bed (muscle fascia, exposed tendons/paratenon, bone/periosteum), patient age, and wound size. Success was demonstrated in difficult cases where other treatment options are limited.
The flexibility that BTM provides to treatment pathways is highlighted, enabling factors such as operating theatre availability and patient compliance to be accommodated.
Of the complications arising, the majority were attributed to patient factors that worsen a patient’s healing capacity (e.g. diabetes and peripheral vascular disease). Of note, the authors state that the method used to document complications was over-cautious because a patient with infection and complete skin graft integration was still included, despite a successful clinical outcome.
Valuable learnings regarding the use BTM are included, such as the need for judicious debridement before application, regular wound reviews after application, and the use of wound swabs and appropriate antibiotics.
The authors conclude:
“BTM continues to show promise as an additional tool in the reconstructive ladder, especially in wound beds less amenable to immediate grafting or soft tissue reconstruction.
Additionally, it plays a role in patients not suitable for autologous reconstruction due to fragility, comorbidities or anaesthetic risk for long procedures.
…BTM is robust and complications, such as infection, can often be strategically managed with judicious care and still lead to positive outcomes.”
Disc: Held in RL(5%) and SM
11/5/2023. loosing height here.. like plane this will bank to left n crash..
revalue $1.20 from $2.00
Growth Return (inc div) 1yr: 29.30% 3yr: -18.31% pa 5yr: 21.50% pa
Value depends on the inflation # trend.
" DCF " model will need reviewing ..will be minus 10% DCF
The Fed Governors are in 'QT ' mode. They want inflation down, down.
A great meeting with DW. It was great to get the reflections and anecdotes across all aspects of the business - so well done to @Strawman for leading a great discussion and drawing everything out of David that we might have hoped to achieve.
Some of my reflections follow.
Competitive Position
DW sounded even more confident that synthetics are winning over biologics. This is supported by the now slower growth at Integra (where the wound division has annual sales of c. $400m), however, Arora is still growing well so the category overall is expanding. (I know there are nuances in the specific applications to different indications, which mean these products are not always going head-to-head.) Ideally, companies like Integra and Aroa - who also have large salesforces - continue to expand the overall market. Then $PNV can ultimately mop it all up. That's the super-bull case, by the way!
R&D
New products (hernia, breast, drug elution) will be over a longer timeframe. PNV used to talk about timeframes, but since the new head of R&D (and Swami) have come onboard, they have stopped doing this. I seem to recall the Head of R&D makeing unscripted remarks at the last AGM where he referred to hernia and breast requiring different mechanical properties. I suspect this means back to the drawing board for the polymer scientists and also there was a reference to need to reach out and network with leading researchers in the field. So I am very much seeing hernia, breast and drug elution as long term "blue sky", which is consistent with $PNV dropping timeframes in their communications.
On capital allocation, it will be interesting to see in the annual results how much is going into R&D. Hopefully, this can be managed in a sustainable fashion as a certain % of revenue.
I'm not concerned by this by the vague timeline for future developments. As David's anecdotes make clear there is huge opportunity for widening the dermal repair applications for BTM and MTX, and progressing the global roll-out. If there can really ramp this up over the next 3-5 years, then $PNV will also have the cashflow to self-fund future developments.
Share Price Action
For what it's worth, I'm not convinced by David's explanation about the SP action being fully-explained by shorts. Shorts are at 2.68% (2-May), vs. their peak of 11.4% mid-last year, and the flow of shares into the market from short selling has been 9,000,000 since the low point in 16-March. With about 30 trading days, that's 300,000 on average per day vs a typical daily volume of c. 2 million.
I think four factors in combination have got us to where we are today:
- David's sale in March
- The market knows they announce the $xm revenue months, and it took a while getting to $6m
- The trading update was a weaker sales number than many bulls (including me) had for their FY23 revenue forecasts
- And yes, 9,000,000 of shorts makes a contribution in the supply-demand balance.
None of this worries me at ths stage. Some quarterly periods will be stronger and some will be weaker. Based on established SP volatility, $PNV is clearly still a trader's stock, so its going to contine to be a volatile ride. But, if you are a long term investor, now could present a great opportunity.
Valuation
Overall, today's meeting leaves me unchanged on conviction (i.e. very strong). I'll review my valuation cases once the FY is in later in the year.
In terms of analysts, 4 of 6 reports on www.marketscreener.com have reduced SP since the recent updates, so the average target SP has gone from $2.53 (close to my central case) to $2.25.
However, this warrants a further look. because one valuation has gone from at or above $1.90 to $0.90. In my view there is nothing in the recent disclosures that makes me think that is plausible (even my Bear case is $1.63). So I wonder if any StrawPeople have seen this report? Ignoring this oddball result, the target has nudged down only very slightly.
For now, I'm happy for the team at $PNV to crack with their great work!
Disc: Held IRL and SM
I wasn’t expecting this! The market doesn’t like it either…down nearly 9%. Explains why the share price has been coming off the boil lately. Plenty of emails from DW while the price was heading up. I notice the inbox is strangely quiet today. Just an observation…don’t read anything into it.
See Announcement
“Chairman Mr David Williams has sold 4.75m shares, the proceeds of which will part settle a US property purchase. Mr Williams still holds 21,384,432 fully paid ordinary shares and does not intend to sell more shares for the foreseeable future.”
David's buying a house in the US and has offloaded 4.75m shares. Ouch!
My first thought is, of all his potential sources of capital, given that he offloaded $PNV, what does that say about his view of the near-term propsects of the company's progression?
Disc: Held in RL and SM
FULL TEXT
Chairman Mr David Williams has sold 4.75m shares, the proceeds of which will part settle a US property purchase. Mr Williams still holds 21,384,432 fully paid ordinary shares and does not intend to sell more shares for the foreseeable future. This announcement has been authorised by PolyNovo Company Secretary Jan-Marcel Gielen.
Just dropped
from Linked In Professor Toby Coates:
On behalf of Beta Cell Technologies and our collaborating partners, I am delighted to announce the online publication of our pre-clinical studies showing the Biodegradable Temporizing Matrix (BTM, Novosorb) supporting survival of pancreatic islet cells in a completely new intracutaneous transplant site outside of the liver in the American Diabetes Association flagship Journal Diabetes.
In this work, we show for the first time survival and function of pancreatic islets when implanted into a unique fully-vascularised alternative site in the skin. The key advance here is the creation of a dense vascular network in the “intracutaneous” site, that is capable of supplying blood to the islet cells and allowing them to survive for up to three months.
Our work shows human islet survival and function in the novel intracutaneous site outside of the liver for the first time.
These studies are important because they are the first step towards creating an alternative islet transplant site, where cells can be monitored for function and rejection, which will be applicable for new sources of insulin-secreting tissues (eg xenogeneic /stem cell-derived beta cells) to be transplanted.
With this work, we fulfill the dream of Dr P. Watson Williams who first attempted to implant pancreatic tissue into the skin to treat Diabetes nearly 130 years ago in 1894.
This work also provides the pre-clinical supporting data for a clinical trial of this promising alternative site. We would like to thank our key collaborators for this work - JDRF International, The Hospital Research Foundation Group and PolyNovo Limited.
Beta Cell Technologies looks forward to sharing our human clinical trial data in the next 18 months.
READ THE FULL PUBLICATION HERE
https://lnkd.in/g5Yzkaiy
JDRF International The Hospital Research Foundation Group
JDRF Australia Central Adelaide Royal Adelaide Hospital University of Adelaide Kidney Transplant & Diabetes Research AustraliaBeta Cell Technologies
PolyNovo Limited
There was nothing material from this afternoon's call to share. However, there are some insights that I found helpful.
US
- Strong momentum sustaining
- Question on Integra feeling the heat and cutting prices. DW batted this away.
Europe
- David Williams (DW) reported that both UK/Ire and Germany (Ger/Aus/Swi/Belg) are starting to get "real traction", after slow starts over the last two years. "They'll soon be bigger than ANZ."
India
- Selling hasn't started yet as there are various administrative items being finalised ("next few weeks")
- 3 Centres of Excellence have signed up; 25 more identified. These will be key to training surgeons
- S&M staff of 20 identified covering the major geogrpaphic areas; plus 2 senior leaders
- India will be used to enrol patients in the US BARDA trial, thereby accelerating its outcome
- Surgeon trials have started with good results so far
- India is "pure market development" - the biologics aren't there
- Pricing will be "market appropriate" (Swami knows what he is doing from years in medical devices in developing and developed markets with J&J. Margins are huge, so they have room to move.)
ROW Overall
- Consequently, should see continued strong ROW growth (currently >100% p.a.) from an increasing base
Employee Expenses
- Due to headcount ramp-up, FY employee expense expected to be just over 100% of PCP
- This excludes the exceptional share based costs from FY22 (referred to in the slides and my earlier straw)
DFU (Diabetic Foot Ulcer) Trial (Last ASX announcement)
- Suspension of enrolment is needed to make sure that the study protocol correctly maps to the standard of care
- It sounds like this is an intervention to tweak the protocol to make sure the results are valid. (To me it sounds like a case of a "stitch in time saves nine.")
Broadening Clinincal Applications for BTM
- Swami's key focus is how to figure out the right way to take BTM to other places in the hospital
- Individual surgeons are innovating, (e.g., BTM being use to save limbs in trauma treatment)
- But this then has to be turned into a model that the salesforce can disseminate
- Trauma and reconstruction are big future categories (but it will be a longer burn)
R&D: Hernia and Breast
- Swami is working to ensure that $PNV accesses the right development experts (outside the company)
- He remains convinced that both hernia and breast applications will emerge, but "R&D is never and slam-dunk, so we can't say for certain"
- I believe we are now starting to get some realism into the discussion on developments. I never trusted the earlier notional timelines, and this is yet another benefit of getting a seasoned industry executive in place
Capex
- Likely to be c. $1m for full year
- More in FY24 and FY25 for the new manufacturing units which expand 2-shift capacity from $120m annual sales to $500m
- Details per capital raise documents
Cash Flow
- Expect to be close to breakeven for the FY; however, management are strongly prioritising growth and will continue to
Closing Remarks (DW)
- We did 10,000 patients in FY22
- We did 8,000 patient in the first half of FY23
- We will eventally be talking about "hundreds of thousands of patients" (SR nodding)
- "We've only just begun" (cue The Carpenters, slow fade)
Overall Impressions
- David, Swami and Jan all appeared to work well together
- This was a well organised, well executed presentation, without descending into endless adhoc anecdotes and answering every granular question
- 15-20 minutes of presentation and a solid 40 minutes on Q&A
- Great job team. I'm so glad $PNV has a CEO again.
Valuation
- I will wait to update my model until the FY.
- With the acceleration investment, I need to see more data on revenue and costs over time
- From a quick check, nothing is out of whack. So happy with $2.46 for now.
Disc: Held
$PNV today released 1H FY23 Results ahead of the afternoon's analyst call. Note that top-line revenue numbers have been pre-released. As usual, this straw includes their highlights, my analysis and key takeaways.
Their Highlights
PolyNovo’s ASX release on 16 January 2023 pointed to record sales growth of 67.5%.
The half year audited results attached to this release show:
• Record 1H FY23 sales of A$27.3m up 67.5% on STLY of A$16.3m
• Total revenue including BARDA of A$29.5m up 62.2% on STLY of A$18.2m
• Strong growth in U.S. achieving 1H FY23 record sales of A$22.8m up 61.0% vs. STLY of A$14.1m
• ROW sales of A$4.5m up by 110.1% vs. STLY A$2.1m including new sales in Hong Kong and Canada.
• The Group recorded a net loss after tax of A$3.8m (1H FY22: A$1.6m profit). The profit in 1H FY22 included the reversal of A$4.7m in share awards and share options expense forfeited by the previous CEO and COO on their resignations and an unrealised forex gain of A$0.4m. Excluding these items results in an underlying loss of A$2.5m for the prior period.
During the Period, the Company’s other key initiatives and achievements include:
• A $53,000,000 capital raising
• First $5 million BTM sales month in September (Sep 2022: $5,402,454) recurring in October ($5,263,100) and December ($5,306,540)
• Appointment of CEO Swami Raote
• Received FDA 510(k) clearance for NovoSorb MTX
• Entered Hong Kong, India, and Canada markets in December
• Leased the adjacent property in Port Melbourne to significantly increase manufacturing capacity
• Commenced SynPath Diabetic Foot Ulcer Clinical Trial
• Awarded Victorian Government grant for manufacturing Diabetic Foot Ulcer product (SynPath)
• Increasing sales teams and customer base globally
• Produced for 1H FY23 the equivalent of 74% of the total devices produced in FY22
Analysis
Revenue
On revenue, these were pre-released, so there was no material new information. A breakdown of the monthly sales following the first $5m month in September, shows that both October and December exceeded $5m.
In terms of revenue drivers, Figure 1 (Slide 5) gives a good overview. Growth in Customers (Hospitals) and Employees (a large portion being sales and marketing) are leading indicators of future revenue growth, as it takes time for the sales to grow once both a new account opens and a new salesperson is in place. This should give confidence of a strong trajectory through H2. The slide makes the point that a return to >60% revenue growth marks the return to better customer access post-COVID.
Figure 1 (Slide 5)

Figure 2 (Slide 6)

On Figure 2 (above), I see that ANZ and RoW are now starting to become significant. At $4.6m in aggregate, they are about the same as the USA was in 1H FY19. While ANZ is relatively mature, it is still growing at >60% and we are yet to see meaningful contributions from Canada, Hong Kong and India. Interestingly, there is no mention of Europe beyond UK. So RoW should be expected to maintain strong growth as we start to get early traction in new territories. BARDA is also growing, so I wonder how far off we are from seeing a strategic stocking decision?
Costs
Today is all about understanding how expensive the cost of accelerated expansion is going to be.
Comparing 1H23 to 1H22, the incremental revenue was $11.2m, while incremental costs added are $16.9m. That sounds pretty bad on the face of it. However, the previous period included a credit of $4.7m reversing lapsed options of departing executives, so the underlying incremental cost is only $12.2m, by my calculation. While I am not a fan of underyling corrections - particularly when they happen every period - in this case, I hope we will see some management stability and so I'll let that adjustment pass. Overall, cost increases will always run ahead of revenue growth. So, the real test of this strategy is going to be in the FY23 and 1H FY24 reports.
Of course, it is important to remember that we haven’t seen the full effect of the step-up in cost base as the changes in increased staffing occurred through the half. We’ll see the full effect in the H2 numbers at FY. I expect to hear some questions on this from the analysts on this afternoon’s call about a forward view on cost evolution.
Profit and Cash Flow
Unexpectedly, therefore, we’ve seen a small increase in losses to $(2.2)m.
FCF was $(3.1)m compared with $(3.7)m in the PCP (note: I'm deducting lease costs from the FinCF line from OpCF), so in the context of now having $50m in cash available, the cash burn is sustainable. Of course, in FY24 and FY25 we are going to see a step-up in capex, as new facilities are built. However, if revenue continues to grow at current rates, the cash generation should mean that cash reserves are not eaten into that much.
My Takeaways
With revenue numbers pre-released, and the capital raise clearly indicating that SR had moved DW on from the “boot-strapping” strategy (no doubt a condition of SR’s joining), today was always going to be about how much costs are stepping up to drive expansion. Because of timing, we are only getting a first view of that.
Overall, it’s a bare-bones, no-nonsense presentation, which I personally find refreshing. It will be interesting to see how it is received. The analysts have been used to getting more granular detail, so I expect there will be more digging in the Q&A, as analysts try to get their FY numbers right. (I’m looking forward to the tussle!)
I am happy with the progress shown in this report. Nothing has a material bearing on my valuation, which sits at $2.46 (being the average of distinct bull and bear case DCF scenarios.) I will wait to do an industry read across including Aroa, Integra and Avita reports to see if this offer any insights on competitive positioning over the coming weeks.
Disc: Held IRL ( 6.8%) and SM (22.9%)
Those following $PNV will be aware that as part of the December capital raise, there was a $3m component of Director contributions. We are all used to DW buying shares in the company and, at the time, I assumed he would be putting his hand in his wallet again.
However, today we got to see who bought what. (Personally, I don't know if this detail was disclosed at the EGM, as I wasn't paying attention. Anyway it is news to me.)
- David Williams $2.229m
- Bruce Rathie $0.380m
- Andrew Lumsden $0.095m
- Christine Emmanuek $0.266m
- Robyn Elliot $0.029m
David aside, these are significant increases in the personal holdings of several of the directors, which I take as encouraging. I am particularly encouraged because this comes about half a year after Swami has joined as the CEO, so for the Board to be feeling good about the company to this extent indicates to me that internal alignment on strategy and execution is there. It is not a big deal, but a positive sign, I think.
What would put the icing on the cake would be for the CEO to get with the program!
Disc: Held IRL (6.7%) SM (23.5%)
PNV - looks all set to re-enter the asx 200. Index funds are likely to buy an estimated $67 million dollars worth of shares.
Interestingly the article places short position around 2.5% of the stock. Looks like most shorts have been able to make a graceful exit without a massive short squeeze.
PolyNovo set to re-enter ASX 200 - The Australian.pdf
Today Polynovo Chairman, David Williams, shared an article by email (below) which appears in the Herald Sun today (subscribers only). While there is nothing new in the story, it is a great read and very good publicity for Polynovo.
I have been taking some profits on Polynovo on Strawman and have sold all IRL. I might regret selling as the share price seems to be caught in an updraft! I really like Polynovo and have even bought some for my mother’s retirement portfolio which she still holds.
I hope to buy back IRL when investors are less enthusiastic. I just think it is now priced for extraordinary growth, which it may very well achieve in the not so distant future.
Disc: Held SM,
Skin in the game PolyNovo half year sales - The Australian.pdf
New release from biotech daily sent through from David Williams this morning. Does help to put some context to the Aus Biotech space. 2 of my biggest biotech holdings did very well in 2022 PNV and NEU and I anticipate an even better 2023.
BOT even got an honourable mention although I don’t agree with the lumping together with the CBD space. This is a biotech using synthetic CBD for dermatology and anti-biotic clinical trials plus it has a new non-CBD lead drug that is due for FDA approval in Q3 2023. I feel this lumping has meant BOT is one of the most overlooked and poorly understood biotech companies on the ASX.
An interesting summary below in BIOTECH DAILY



Details of the $PNV capital raising now out.
also a detailed presentation.
Raise is:
- $30m institutional (+2.4% shares)
- $3.0m Director shares, subject to EGM in January
- SPP up to $17.0m for retail shareholders.
So the raising represents a dilution of <5%.
Once again David is showing total alignment. I assume he will be providing the lion's share of the Director contribution.
Looks like the insitutional bit is done, as it comes out of trading halt tomorrow.
Rationale is about organic acceleration: sales & marketing, R&D, new indications, and production facilities to support $500m sales. (that's more than 5x consensus 2024 sales!)
My conclusion: Good idea. Boot-strapping off the smell of an oily rag would be a drag on growth. Go big or go home.
Details on the rationale (from the release)
Accelerating PolyNovo’s growth ambitions PolyNovo is well placed to achieve significant growth in the near-term with four key vectors driving execution of the growth plan:
• Geographic and sales team expansion: Ongoing growth in core markets, including continued expansion in US presence with 54% increase in US staff over FY23F while simultaneously entering Canada, Hong Kong and India, with revenue expected from CY22. Exploratory steps have been taken to facilitate entry into China and Japan – the global #2 and #3 medical device markets
• New indications for NovoSorb: NovoSorb BTM is already a leader in third degree burns in Australia and on a steep growth curve in US burns. There is a significant opportunity to increase TAM through access to new markets with existing products. Surgeon led insights and innovation are driving PolyNovo to work with clinicians and regulators for new indications and applications
• New products: Investing in R&D capabilities to support new product ranges as well as upstream application and marketing, insight generation, biologic, pre-clinical sciences, process and package engineering. Potential for alliances with global category leaders and academia for Clinical & Health-economics evidence. PolyNovo sees an opportunity to enter orthobiologics, breast reconstruction and fascia repair
• Capacity expansion to satisfy growth: PolyNovo will commence development of a new colocated facility with production, R&D and office facilities. The facility will be designed to support an additional A$500m in revenue a focus on flexibility, modularity and automation. Total expected build cost of A$25m, with spend predominantly incurred in FY24F
Disc: Held in RL and SM
07/10/2022
This is my first attempt at valuing Polynovo on Strawman. I find valuing pre-profit businesses very challenging. However I think that Polynovo will eventually have a large share of the total addressable burns treatment market within a decade, as well as a number of other novel uses of BTM. What value do you put on that? However, I think the risks are also very high, eg. competitive treatments, failure of novel treatments, expiring patents etc.
There are also polar views amongst investors and brokers on what the business is worth. Large short positions are having a huge impact in the short-term resulting in manic swings in the share price.
Broker Views
Phil King, founder of Regal Partners, is possibly the most bearish on Polynovo. Phil King’ named Polynovo as his top short idea at the 2019 Sohn Hearts & Minds conference. I wouldn’t be surprised if Phil King is still very short on Polynovo.
Wilsons is underweight on Polynovo with a $1.11 share price target. Wilson said “At $1.62 per share (18.1x FY23e EV/Revenue) PNV is 3-4x more expensive than most product-relevant benchmarks we reference. Aroa (ARX; O/W $1.58 PT), Avita Medical (AVH; M/W $1.74 PT) and Integra (IART; not rated) trade at 2-4x 12-month forward EV/Revenue multiples.”
Price/Sales is a very crude way of valuing businesses because is does not consider profit margins. When Polynovo relies less on the sales team and builds trust within medical circles, the profit margins will be very high. I don’t think this will take long due to the ripple effect.
According to Simply Wall Street the average price target for Polynovo from 6 analysts on the 30 August 2022 was $1.68. Since then Polynovo has announced a new record month with sales over $5 million.
My Valuation
If you work on Polynovo achieving an ROE, or internal earnings growth of 85% over the next 5 years, and you were looking for a minimum 10% return on your investment, Polynovo would be worth $1.95, say $2.00 (using McNiven’s StockVal formula starting with shareholder equity of 3cps, APC of 85% and with all earnings reinvested into compounding future growth).
I don’t think this is unrealistic when you consider the ‘Rest of World’ revenue growth for the recent quarter was 84% and the runway for growth is huge!
At the same time I think the risks are high and I will possibly take some profits along the way to reduce my exposure.
Disc: Held IRL (3%), Strawman (15%)
Edit: corrected monthly growth in sales past 8 months to 4%, not 4.9% as previously stated.
A very pleasing Q1 FY23 sales result, September quarterly global sales up 73.3% on the same time last year.
The monthly sales graph provided in the announcement was a bit weird, if I have interpreted it correctly! The ‘x’ axis does not show consistent time periods between the monthly sales bars. This skews the graph to make monthly sales growth look linear, which it is not!
Polynovo have done themselves a disservice here. What this graph fails to show is the accelerated monthly sales growth over the past 8 months up to September 2022 compared to the previous 3 periods.
On the graph below I have indicated the time gap in months between each of the monthly sales bars, and calculated the monthly growth in sales for each period (ie. simple monthly growth for the period, not compounded month on month growth).
In the first 8 month period on the graph (Apr 2019 to Dec 2019) the growth in monthly sales was 7.7% per month.
Then for the next 18 months (Dec 2019 to June 2021) the growth in monthly sales was much slower at about 2.1% per month.
Between June 2021 and Jan 2022 (7 months) monthly sales growth increased to 3.4% per month.
The good news is that over the past 8 months the growth in monthly sales has increased by 4% per month. This acceleration in monthly sales growth is very significant for Polynovo, especially if it continues at this rate for the remainder of FY23 and beyond.
Disc: Held IRL (3%), Strawman (12.6%)
ASX Announcement: First Ever A$5m sales month and Record First Quarter Sales A$5 million Sales Month
• The Company is buoyed by its first ever $5m sales month and by the growth trajectory outlined below
• The graph highlights the first achievement of each AUD 1m incremental monthly sales milestone

First Quarter Financial Highlights
• The Company had record (unaudited) September quarter sales of AUD 12.5m up 73.3% on STLY of AUD 7.2m. This includes a record month in September of AUD 5.4m.
• Growth accelerated in U.S, achieving a record quarter sales result of AUD 10.4m up 71.3% vs. STLY (in USD $7.3m up by 61.3% vs. STLY), whereas sales ROW at AUD $2.1m grew by 84.0%
Chairman, David Williams said “By focusing on hiring the right talent and expanding our commercial footprint, we are confident of building a Global leader in Soft Tissue Regeneration based in Australia. However, while the growth trajectory is clear and exciting, month to month sales are still lumpy.”
Chief Executive Officer, Swami Raote said “Our results are a vindication of surgeon recognition of consistent outcomes, better patient experience along with hospital systems acknowledgement of lower complexity and cost associated with NovoSorb BTM. I am pleased with how our teams are now beginning to translate our burn heritage and supremacy into trauma and other acute surgical soft tissue reconstruction opportunities. PolyNovo has always been focused, responsible and capital efficient in delivering results and I look forward to accelerating our global impact.”
This announcement has been authorised by PolyNovo Company Secretary Jan-Marcel Gielen.
About PolyNovo®
PolyNovo is a disruptive medical device company, focused on Advanced Wound Care. PolyNovo is an Australian based medical device company that designs, develops, and manufactures dermal regeneration solutions (NovoSorb BTM) using its patented NovoSorb biodegradable polymer technology. Our development program covers Breast Sling, Hernia, and Orthopedic applications. For further information and market presentations see www.polynovo.com
About NovoSorb®
NovoSorb® BTM is a dermal scaffold for the regeneration of the dermis when lost through extensive surgery or burn. NovoSorb® is a novel range of bio-resorbable polymers that can be produced in many formats including, film, fibre, foam, and coatings. NovoSorb’s unique properties provide excellent biocompatibility, control over physical properties, and a programmable bio-resorption profile.
A positive update. September achieves first $5m month at $5.4m .
Q1 sales of $12.5m, up 73.3% on PCP, with US growing by 61.3% on a constant currency basis.
There is a currency tailwind in this overall Q1 number.
Good to see US sales growth has picked up again after softer growth in a couple of the Q4 months.
ROW grew by 84.0%.
(Reaching my target FY23 sales of $70m is probably out of reach, but that supports a SP of $2.46 and I am way ahead of consensus which is $63.5m)
Disc: Held IRL and SM

https://www.businessillustrator.com/product/innovation-distribution-curve-crossing-chasm-cartoons/
Coming from an agricultural background where I specialised in sustainable agriculture and Best Management Practice (BMP) adoption (before retiring), I am familiar with how long it can take for an industry to reach widespread adoption of a proven innovative technology (sometimes longer than 7 years). As a practitioner this phenomena is extremely frustrating…but it is reality!
I thought this might also be the case for innovative medical technology and very relevant to where Polynovo is in its current journey. While researching this online I found an interesting article written by Mike Fix.
Mike Fix has more than 15 years experience across the pharmaceutical and medical device industries, with additional experience in retail. His work focuses on leading engagements assessing market landscapes for startups, mid-size, and multinational medical device manufacturers.
Mike talks about the Rules Of Engagement, and Adoption and Penetration in medical devices.
Mike believes “The average clinician takes no personal stake in helping a technology succeed or fail; their only stake is in providing positive outcomes for their patients. To convince them it’s worth using a new innovation in favor of what they already know works, you need to motivate them through belief that the technology will deliver:
- Improved patient outcomes, safety, quality of life or comfort
- Improved efficiencies, economics, or time savings
He says “Adoption follows a well-understood path to standard of care. Most technologies fail to get past the early adopters (about 15 percent) through the classic “chasm” to the early majority, which serves as the gate to the rest of the market.”

“The difference between each adoption group is its intrinsic beliefs about both the status quo and what the new technology offers. The innovators and early adopters are driven primarily by the idea and promise of the new technology. Therefore, they need little evidence or guidance on patient selection; they want to figure it out on their own, and often believe other clinicians think like they do. In contrast, the “early majority” needs hard evidence and a proven patient selection algorithm. And, importantly, the “late majority” and “laggards” share the early majority’s needs, plus personal experiences that give them confidence in the new technology”, he said.
“Consequently, convincing each subsequent stage of adopters requires more evidence and validation of a new technology’s clinical and economic value proposition, along with a roadmap identifying the best patients. If no guidance is provided on best-use case scenarios (i.e., patient segments most likely to experience a positive outcome from the treatment option), physicians usually apply the therapy to patients as a last resort. As a result, outcomes will be questionable, at best, and often negative — which ultimately diminishes adoption rates.”
“However, if a pipeline of evidence and patient selection guidance is provided, then early stage adopters will gain confidence, share their experiences, and provide useful insights. These insights will help the manufacturer make relevant modifications to the product and/or resolve other adoption-related barriers, enabling the manufacturer to continue building confidence among physicians, and to roll the therapy up and over the adoption curve.”

Mike says “Once physicians are confident in a technology’s value proposition for a certain segment, they will more readily expand to a fuller indication. Each positive step accelerates and deepens market penetration. Full adoption occurs when behavior change fully transitions. The clearer the benefit and better the results, the faster the adoption.”

Mike goes on to talk about the importance of patient selection and segmentation in the early stages of adoption. However, the point I wanted to make here is that Polynovo is still early on Roger’s Innovative Adoption Curve, and I believe just starting on the journey between T1 and T2 in the Adoption Path above. We know this because the revenue growth rate is not exponential (yet!).
I think new CEO, Swami Raote, is the right person at the right time to lead Polynovo across the Adoption Chasm. Swami said recently “When I think about spreading this tech, I think about the access, secondly education and then thirdly, how to scale it up. To get a hospital [as a customer], you need one surgeon to believe in the product. And then they spread it to more surgeons.”
Currently, I think Polynovo is priced for revenue growth of c. 70% (Using McNiven’s Formula), and when the share price reached $2.25 recently it was priced for 100% revenue growth. It was revealed last week that FY22 revenue growth was 43% and obviously the market was expecting a lot more.
However, if Polynovo does cross the Adoption Chasm (and I think it will) the current revenue growth will be irrelevant as sales will grow exponentially between T1 and T2 on the adoption pathway. I think the million dollar question is when will this happen?
Disc: Held Strawman (11%) and IRL (2%) after a significant reduction between $1.80 and $2.20.
References:
Take The Narrow Path To Wide Adoption — Here's Why And How
https://www.afr.com/technology/polynovo-shares-slide-13pc-after-earnings-miss-20220826-p5bczz
Research update from Bell Potter received with morning from $PNV Investor Relations
TP upgraded significantly from $1.50 to $1.90.
(I had a few helpings yesterday, so I need to stop here to avoid indigestion.)
Polynovo (PNV)
Matrix Revelations
FY22 result. Record revenue of $41.9m represents a 42% increase from FY21 (BPe $43.0m). Operating expenses of $41.1m were 26% greater than FY21 (BPe $42.1m). Net loss of $1.3m reflects improvement since FY21 (net loss $4.9m). Adjusted NPAT of $0.2m accounts for the $1.4m impairment loss on the sale & leaseback of Port Melbourne facility. Capital expenditure of $0.5m (BPe $1.5m) and net cash outflow of $1.6m (BPe $0.9m). Cash position of $6.1m as at 30 June 2022.
Matrix expansion & strengthening portfolio. BTM sales recorded in new territories within Europe and follow-on sales in key markets (India, Taiwan, South Africa) within the burns & wound care sector. The new MTX product allows application in complex wounds over joints and ulcers (venous leg, diabetic foot). FDA 510(k) clearance is targeted in 1H23 with commercialisation in 2H23. R&D continues within breast & hernia and novel therapeutics including the BetaCell collaboration for treatment of Type 1 Diabetes.
Clinical trials update. Enrolment of 23/120 patients in the pivotal BARDA funded trial in full-thickness burns. SynPath diabetic foot ulcer trial has commenced enrolment in August 2022. Expected completion for both studies is 4Q23. Randomised controlled trial also underway with Flinders University in neuroischaemic diabetic foot wounds.
Investment view: Maintain Buy, Price Target $1.90. We increase our price target to $1.90. The valuation has been generated from a blend of two methodologies: DCF (WACC 10.5%, TGR 3%) and EV/Revenue (8.6x from comps). Key catalysts driving this upgrade include BTM expansion in the Asian market, greater penetration within the US market from existing & new accounts and launch of MTX during 2H23. Product pipeline with SynPath and the novel therapeutics also strengthen the longer term potential for Polynovo.
Recommendation:
Buy
Previous Close:
$1.64
Price Target:
$1.90
Bell Potter Securities Limited
Level 38, 88 Phillip Street,
Sydney NSW 2000
Disc: Held in RL and SM
Good results – and the market reaction so predictable!
$PNV yesterday reported its FY22 results. While overall revenue growth of 42.8% to $41.9m was a touch under consensus ($42.2m, marketscreener.com., n=6 and below my bullish view of $43-$48m), eps and cashflow were slightly negative, where consensus was for both to get into positive territory. None of this concerns me but it explains the market reaction.
In this Straw will cover:
1) the list the highlights from the results
2) details from the discussion on the investor call and
3) my overall take-aways, which includes an assessment of the market reaction which was a 19% drop in the share price!
1) FY22 HIGHLIGHTS
The full highlights of FY22 are (with comparisons to FY21)
- Revenue up 42.8% at $41.9m
- Novosorb BTM sales up 47.6% to $37.6m with USA up 55.1% at $32.1m
- Customer accounts increased by 135 hospitals, excluding distributors
- Key appointments delivered: CEO and Ops Director
- Staff grown from 106 to 152
- US sales team now at 54. There are 9 new starters in last two months and 5 positions remaining unfilled
- R&D team expanded from 1 to 8 (or 9) to intensify new product development, with R&D spend increasing from $3.6m to $5.7m
- 510(k) lodged for MTX with FDA
- BTM registration lodged in Canada. Sales to some surgeons have started and training of staff has commenced.
- Recruitment underway in 3 clinical trials: BARDA Pivotal; US chronic wound insurance reimbursement; and chronic wound study
- $0.62m BTM contributed to clinical trial and $0.52m BTM for humanitarian causes
- Several European markets entered; 3PL deal struck in Belgium
- Property sale and lease back completed
- $3m debt paid down
2) DETAILS OF THE INVESTOR CALL DISCUSSION
Conduct of the Results Call
Those who know Chairman David Williams’ style, appreciate his maverick, straight-talking and unconventional approach. But today’s call bordered on the chaotic. David raced through the slides. The financials weren’t even properly presented. David’s introduction was then followed by a loosely structured conversation with CEO Swami Raote, former Acting CEO Max Johnston, and CFO Jan Gielen all chipping in on various topics.
It was always going to be an interesting call to choreograph but, from my perspective, it needs to be the last one like this. At 1H23 David needs to let Swami take the lead. In fairness to David, a lot of information was conveyed in the discussion which you’ll see below, and we got to see more of the emerging thoughts from Swami that will hopefully set the future direction of the company.
US Sales
The analysts picked up (and prised open in the Q&A) that in the USA, May and June were slower compared with a record April. July was strong again and August is “tracking well”. This signals a slowing of the growth rate in Q4 (and explains why results missed my estimate, which was ahead of consensus.) US growth in H2 was 23% up on H1 (which is 51% annualised), compared with up 55% for the year. While that sounds like splitting hairs, all the rhetoric in previous presentations had conditioned the market to expect accelerating growth in the US. For me, what perhaps made the US number surprising was that H1 was more impacted by COVID-19 access issues than H2, although DW said that the US team had used remote engagement successfully when access was constrained.
Growth in new Accounts (hospitals):
Global new accounts (hospitals) added accelerated. 135 hospitals were added in FY22 compared with 99 hospitals added in FY21. Of these, 80 were added in the US. Sales in accounts existing at the start of the year grew by 88%. This is consistemt with improved access post-COVID and a much-expanded S&M team.
ANZ bounced back after lockdowns impacted H1
ANZ was impacted by lockdowns in H1 (with NZ impacted into H2). With lockdowns lifted, H2 sales were 63% up on H1. The ANZ sales team has now been expanded with a dedicated person in NZ. In 2021 it was over-stretched with one rep covering 30 accounts. Now, the senior reps are supported by associates, a model which is working well and creating entry-level opportunities.
ROW – what’s going on?
It’s a tale of two very difference parts. ROW consists of UK&I and what is now quite a long list of continental European countries. Overall, sales were up 50% from $1.6m to $2.4m, as follows:
- In UK&I the direct sales model is getting traction. UK and Ireland achieved sales of $1.3m, up 185% y-o-y from $0.5m. 31 new accounts added from a salesforce expended to 9 from 5.
- The Rest of “ROW” (Continental Europe) was flat: Backing this out, all these other countries achieved $1.1m, essentially flat from FY21, but apparently it “improved in 2H22”. (I asked a question why the distributor model in Europe is clearly not getting traction, but this question was not selected in the Q&A! :-(
Capex down to $0.5m from $3.6m
With the second production line built and undergoing validation, capex has fallen significantly. It was emphasised that this is a capital-light business, and they are keeping spare production capacity in place to underwrite future growth. FY23 capex will also be low.
BARDA
BARDA – the US government agency – is funding a $15m multi-year trial to generate the data that, if successful, will lead to $PNV being stockpiled by the US Government, so that there are strategic reserves in case of a major disaster. Swami has experience from J&J in working with BARDA and he said that the focus and attention BARDA are giving to BTM is as high as he has ever seen (including on vaccines!) Down the track, this offers the potential for some material boosts to sales. BARDA is working with $PNV and the FDA to get approval for BTM for second-degree burns. This would give a more general market-wide boost to BTM sales.
Product Development
Good progress on all 3 “pillars”
- Advanced Wound Care: Focussed on extending the current product beyond burns. The idea is to improve connections with innovative surgeons who are surging ahead. R&D will work to tailor the product to their needs (e.g., smaller sizes; useful shapes).
- Implantables: 4 prototypes have been developed for hermia; and one “breast sling”. No specifics provided on timing for launch.
- Therapeutics: there is “enormous interest” by external partners (pharmaceuticals) to use drug-impregnated BTM as a local drug delivery vehicle. David and Swami recalled a recent trial case study from Adelaide in treatment of a Type I diabetes patient, which was transformational for the patient. David in his latest red jacket, provided his characteristic simulation of the procedure. (My note: If this gets traction, the opportunity is huge, but we need to think of timelines in terms of 7-10-+ years, but it does present very material, long-term blue-sky).
The focus in the short to medium term will very much be on Advanced Wound Care, because the regulatory hurdles are lower, time and cost to market is lower, and the synergy with the existing sales and marketing capability is very high. Several clinical studies are being funded to get the data to support a broader set of wound indications. The sales reps need this data to influence their customers.
Finances
There is $6m cash on hand, down only $1.1m from YE21. Last year $PNV said “The business will continue to reinvest cashflows to expand market share in existing markets, enter new markets, and develop new products.” That is exactly what they have done. In addition, $3m of debt has been paid down using proceeds from the property sale and lease back.
I think this is where the market might have been expecting more. Looking back at FY21 results (slides 28 and 29), the net profit and cash flow trend charts set the expectation (reflected in consensus forecasts) that both cash flow and net profit would be clearly in positive territory. Neither were.
That said, H2 was operating cash flow positive compared with H1 s;ightly negative.
In Q&A, when asked to comment on the sufficiency of capital, DW was more circumspect than this time last year, deferring to Swami. That was a good call because he has to leave space for Swami to put forward his growth strategy, To close the option of access to capital would have been a BIG MISTAKE. IF Swami is smart (and I think he is) he would have agreed this with the Board as a condition of joining. Of course, with debt paid down and rapidly growing revenues, $PNV has greater debt capacity in FY23.
Jan reported that margins were continuing to increase, due to price increases to offset rising costs and higher facility throughput. He reported gross margins are up 0.2% to around 95%. There was a brief discussion that the market can bear higher prices if necessary, because the competing products are more expensive and their treatment and post-treatment support is more complex and therefore more costly. The healthcare economics equation is about more than just the product cost.
Sales and Marketing – further insights
The imminent Canadian launch will adopt a hybrid sales and marketing model. A distributor partner has been selected with 17 identified, experienced sales agents. Ed, the SVP S&M Americas will retain responsibility for the sales in Canada, including training and supporting the outsource partner. David, Swami and Max were all very positive about the prospects for this model, and they expect rapid traction in FY23 as a result. Swami said that some surgeons in Canada who have already carried out some procedures have reported "tremendous results". Surgeons are already placing orders “at their own risk” ahead of product registration.
Generally, we heard repeatedly that sales and marketing for BTM requires a “high support” model. Swami said that many distributor partners wouldn’t work, because they see their job as “getting to the hospital purchasing desk” to take orders. With BTM, the relationship is between the sales agent and the surgeons and nurses. (I think this explains why Europe is treading water at the moment. They know what to do, they just didn’t want to talk about Europe because they don’t have a solution there yet!)
In the USA, Max mentioned that 2 GPO deals are in the pipeline, with one big one close to landing. However, Max and Swami reinforced that GPO deals must be underpinned by the Sales and Marketing people on the ground at the hospitals for the reason discussed above.
On sales and marketing, we heard a repeat of prior messages: that new sales agents don’t do that much in the first 6 months, but that by 12 months they are contributing more than their costs. This time, however, the messages were somewhat more nuanced pointing out that it depends on a range of other factors. These include how many existing accounts are in the territory and how mature they are. Going forward, it will be important for both management and analysts to learn how to model sales growth expectations from S&M investment.
We heard some more of Swami’s ideas
- He believes that BTM is a superior product to biologics: better clinical outcome, easier to use, better economics (for the hospital), better patient experience in treatment. He cited that staff who have come across from Integra and Recell support this.
- He wants to leverage a November Conference in India to stand up a team there and launch. “Eventually, there will be more procedures in India, however US will remain biggest for revenue, due to much lower revenue per procedure.” He doesn’t want to wait to strategize on this, but he wants to get going. DW said it had been discussed at the Board meeting that morning. Swami said that hospital-sourced infections is a big problem in India, so BTM has big advantages because of the reduced risk of infection in its use.
- More generally, Swami is focused on how $PNV can accelerate global adoption, add new markets, broaden from burns into trauma (DFU, oncology, etc.), and move beyond the hospital.
- He believes in the US sales team can “take the trajectory up”.
- He is scanning for acquisitions that can enhance market access. They are not looking for products, but access to surgeons, particularly podiatric surgeons in the US (who usually operate out of stand-alone facilities, not hospitals, unlike in Australia). (Incidentally, DW asked investors to stop writing to him to suggest they buy $AVH or $ARX. They don’t want biologics in the portfolio.)
- He said that if they had a blank sheet of paper his market priorities would be USA, Japan, China, Western Europe. So, I think we should see some changes over the next year. When challenged in Q&A over whether the priority should be to bed down existing markets before expanding, Swami’s view was that this wasn’t necessarily valid, as what goes on in one market doesn’t detract from another. (I believe that Swami will bring to $PNV an impressive contacts book of sales and marketing managers from his international experience, and that this might prove to be a huge asset to $PNV.)
- In terms of product development, he wants $PNV to get even closer to the surgeons and get their feedback on how they want to use the product. He is clearly an advocate of customer-driven innovation. An example of this is regional Australia. He asked, how can procedures and product be evolved to allow the patients to come in to hospital for one treatment, and then not have to return to hospital? Swami cited what he called “unicorn surgeons who have already taken BTM to more places that we have”.
- He noted that applications involving implants will have a longer regulatory hurdle to overcome (lots of experience from J&J!), and he believes that engagement with regulators is very important.
3. MY OVERALL TAKEAWAYS
On the Results
By any measure, these were good results in USA, ANZ and UK&I; even if it is unclear why May and June experienced softer growth in USA. It is not a concern at this stage, because on such a fine timescale you’d reasonably expect some variability month to month.
Europe (distributor markets) requires attention, as the model is not capturing the opportunity. They know it. They just don’t have a plan yet.
They did what they said they would do: using cash generated to grow sales and marketing and expand product development. This is exactly what they should do. In addition, some debt was paid down, creating balance sheet capacity.
The Future
Swami is passionate about the product and the market opportunity, and he wants to move quickly. Given what $PNV has proven to date, it makes sense to go much harder on key markets in Europe and Japan and (I defer to Swami’s view about ...) China.
This is going to take a big investment in building out the global sales and marketing capability. For example, it has already taken three years building in the USA, and they have further to go. It won't be so easy elsewhere, but fortunately, Swami have very relevant experience outside of USA and Europe - a huge asset to the company. It will likely involve a mix of direct selling and deals with appropriate contract sales and marketing entities/distributors. It is unclear to me what the cost of this will be, but $PNV should be willing to raise debt and capital to fund it. Growth at these margins will drive shareholder value.
I expect Swami will go through his own adjustment period. $PNV doesn’t have a fraction of the depth and breadth of capability that Swami was used to in J&J. So, he will have to lead in a very different environment. It is important that David supports him, but also doesn’t stifle him. This is a key potential risk to key an eye on, but David is well-aligned with investors.
Finally, the Market Reaction
In short, the 19% SP drop is entirely unsurprising for the reasons I foreshadowed in my Straw the evening before results.
Since the Q3 trading update, which received a muted SP response, the SP had rallied 77% from $1.13 to close at $2.00 prior to results, standing 22% higher than market consensus. This was driven by DW’s share purchases, buying by short sellers, and a positive response to Swami’s appointment.
The results in terms of revenue, net profit and cash flow were a slight miss on consensus, so it is entirely unsurprising that the price has dropped right back to the consensus level. The market has behaved precisely as you’d expect.
Furthermore, not only is $PNV one of the more shorted stocks on the ASX, its historic volatility makes it a traders favourite. Don't invest in $PNV if you don't have a strong stomch for volatility. We should expect this to continue until performance becomes more predictable. Here, I think David and Swami have a key role to play in improving the quality of investor communications. This subject is worthy of a separate note.
All that said, I believe Mr Market has handed investors another opportunity to get onboard at what I believe is a large discount to fair value.
CONCLUSION
With $PNV now 6% of my portfolio and my high conviction unchanged, I am a HOLD for now.
However, should there be any further SP weakness below $1.60, then I will increase my holding.
I believe the market is significantly under-valuing $PNV. However, thee is real valuation risk in sales and marketing execution. In the appointment of Swami Raote I believe the risk has reduced, and I will be eager to observe how he settles into the role over the next 12 months.
I’m looking forward to the AGM on 28th October and to FY23 – which will hopefully be a “cleaner” year without all the caveats and stories about COVID-19 obscuring the underlying performance of the business.
(My valuation to following in a week or two.)
Disc: Held on SM and IRL
I am very much looking forward to hearing Swami and David deliver the $PNV FY22 Results tomorrow.
Whatever the results, it will be hard to call the price action. Shorts (graph below) have been reducing given positive updates(Q3), DW share buying spree and a strong CEO appointment. However, taking a 3-year view they remain high at 6.78% as of last week - there's a possibility for strong movement either way, depending on response to results.
Analysts don't know what to think or haven't updated. SP is 22% above consensus (n=6, marketscreener.com, range $1.11-1.90). I'm at $2.80 (central case, DCF, will update on SM after I have digested the results).
Consensus on sales is $42m, but my estimate is $43-48m range.
With DW's last purchase reported on 9 June, and with his strong buying through Q4, I assume the sales trajectory will be strong.
Cash flow will be at or close to breakeven, before including proceeds of real estate sale.
A few uncertainties impacting cash flow are impact of wage increases and inventory changes to support sales, although according to Swami $PNV is being sought out in the sector as employer of choice and according to David, supply is easy due to very high %GM, so no need to hold significant local regional stocks now that borders are free flowing again.
Here is the link to the results call, tomorrow at 2:00pm:
https://services.choruscall.com/mediaframe/webcast.html?webcastid=DVUbmPqJ
Disc: Held.
On both SM (18%) and IRL(7.4%), $PNV has become my largest position, even after trimming along the way. My conviction on this firm is such that I am going to let my winners run.
from www.shortman.com.au

PNV new CEO impressions.
Apart from a few technical hitches that was a good quick introduction to the new CEO.
My impression is that David has found a visionary with plenty of experience to help shape PNV into a global success story.
Swami outlined his work history and experience working for J and J across Indonesia, India, Philippines, China and the US. He has worked his way from the ground up in global medical technologies to the ‘head of the table’ – as Global President for J and J Vision Care.
Motivation for coming out of retirement
He describes PNV as a ‘diamond in the rough.’ He stated that he is amazed at the simplicity of the Novasorb technology to help the body to heal itself. This reduced complexity is what excites him as he sees PNV transforming from a burn and wounds company to a complete soft tissue platform company.
1. SIMPLICITY:
The reduced complexity of the technology is its competitive advantage. He describes the value chain as robust and scalable quickly. He describes the limitation of all other competitors to PNV as they will have difficulty growing and scaling due to the complexity of their manufacturing as opposed to PNV.
2. BOARD:
His second stated reason is that he has rarely seen such a unified and motivated Board. They are all unified and motivated to push the company global
3. COMMUNITY:
The teams and staff are all very motivated and excited to be working with the Novosorb technology and there is a real belief in the product
4. MARGINS:
The margins and sales model has huge potential
US
- PNV needs to get key influencers and surgeon advocates on board to sell to other surgeons.
- Retraining surgeons as this technology is totally different from what most of them were taught in their training
- There is initial disbelief and suspicion at how simple and effective the technology is – once a doctor tries it they are ‘converted’ – He want these converted ‘Evangelical’ surgeons teaching others about Novosorb
- Peer to Peer education in the key to PNV sales strategy
EMERGING MARKETS
- PNV will be the inventor of this sales model- He sees the company as constructing the rule book here.
- There is a greater need for the product in the developing world as burns and / trauma is more common
- Swami believes adoption will be quicker and easier here as developing countries are faster at adopting and embracing new technologies.
NEW PRODUCTS
PNV can’t keep pace with the number of case studies and ways doctors are successfully finding to use NOVOSORB.
Once again he reiterates that the technology will move to a complete soft tissue reconstruction platform.
M & A
- Is possible for acquiring supply chains and capable distributors that already have pathways into plastics and reconstruction
- If PNV finds good synergy – with income generating companies there may be future acquisitions
DIRECT V DISTRIBUTOR SALES
-Distributors must add value – not just a warehouse
-They must be involved in surgeon education or else using distributors will not be successful. On this point he does seem very aligned with David Williams
-Big belief that PNV must impact the customer positively
JAPAN
Swami states that Japan is a great market to attack – as it is evidenced based etc… It is a gateway and provides traction into the Asia Pacific markets. As head of Vision Care (Contact lenses etc…) he will have a wealth of knowledge and experience in navigating this traditionally difficult market.
He states he is more cautious about entry into China and Brazil
PARTING COMMENTS
Swami is a big believer in company community. This is an area that has been attacked with PNV over the past and shorters have used this instability to their advantage. There was clearly tension with the departure of Paul Brennan. However hopefully this is the start of a calmer and more unified chapter.
If the team feels good about what they are doing the business will thrive.
It was also confirmed on this call what most shareholders had suspected PNV is involved in taking Novosorb to the Ukraine. Swami mentioned that ED the head of the US sales division is in Ukraine to support surgeons use their product. If that isn’t feel good I don’t know what is.
David finished the call saying that there were lots of things happening right now and that he would be communicating these events with shareholders very soon.
So overall a pretty excited and happy shareholder.
Cheers
Nnyck
Just adding to @Slomo’s straw, it will be particularly interesting to hear what CEO Swami Raoti has in mind for the the future direction of the business. Here are a list of questions Chairman David Williams will be asking Swami in 30mins from now:

Webcast Registration: 9.30am, 3 August (today!)
Today Chairman David Williams shared a report from Macquarie Research dated 6 April 2022. PolynovopnvAu-SalesMomentumBuilding.pdf
Macquarie gives Polynovo an ‘Outperform’ rating and a 12 month price target of $1.60. Macquarie expects BTM sales of A$25.7 million for 1H23, which would be a 58% increase on 1H22.

Disc: Held IRL and SM
Polynovo Chairman, David Williams, shared two broker notes and price targets for Polynovo this morning by email. Bell Potter have recommenced coverage of PNV.

PolyNovo’s (PNV) 3Q22 trading update had group revenue of A$12.3m, +59% vs pcp. But there appears to be a step down in US BTM sales, with Feb/Mar average implying ~A$2.9m sales per month compared to A$3.7m in Jan-22, although exit rate is unclear. Despite this, PNV has achieved YTD group revenue of A$30.42m, and applying 3Q22 revenue to 4Q22, the result appears in line with FY22CL group revenue of A$42m. Cash end-Mar-22 ex-property sale was A$3.8m (+A$517k vs Dec-21), a positive outcome given this was a key concern for investors at its 1H22 result. With the result tracking in line with CLSAe, we make no changes to earnings and reiterate our A$1.80 price target and BUY rating.
Our PNV thesis: what’s changed following PNV’s 3Q22 update?
We’ve held the view that 2H22 would provide a platform for PNV to leverage off its growing sales force and recent sales momentum, and cash flows end FY22 flat H/H (ex-property sale), alleviating concerns of the need for a near-term capital raise (noting we do see the value in additional funding to fast-track its R&D portfolio). This transition could support a pathway to profitability from FY23 onwards. PNV’s trading update appears to support this journey, with group revenue in line with FY22CL albeit on what appears to be step down in US BTM sales. And with cash increasing QoQ ex-property sale, the latter adding a buffer of ~A$3m after paying down debt, for now we maintain our longer-term thesis on the stock.
BTM sales in line with CLSAe, albeit below strong Dec/Jan result
US BTM sales were A$9.53m for 3Q22, +79% vs pcp, but the result implied a step down in Feb/Mar average revenue following strong Dec/Jan results, and provided no clarity on the exit rate. Despite this, PNV added 15 US accounts in the quarter to 169 total, suggesting it continues to gain traction on the ground, but needs to convert these higher penetration rates. Australia and New Zealand saw strong gains, with 3Q22 sales of A$1.16m, suggesting a stronger than expected exit rate.
QoQ rise in cash balance supports view of FY23 cash flow positive
Commentary from PNV at its 1H22 result suggested 2H22 cash balance would be flat H/H, ex the A$6.35m property sale. But with PNV’s cash flow adding +A$517k QoQ, albeit with no clarity on how this was achieved, the result should help see PNV’s cash balance turn cash flow positive in FY23, in line with CLSAe.
Price target A$1.80 and BUY rating retained
While Covid disruptions may still affect near-term sales, BTM sales and account growth show momentum is being maintained. Near-term share price volatility is likely to persist, but longer-term value remains

Polynovo (PNV)
Cash and Burns
Novel device in burns & complex wounds market
· Polynovo is a commercial stage medical device company and its core offering, NovoSorb Biodegradable Temporising Matrix (BTM), is used in the management of burns and complex wounds.
· Expanding indications for use have increased its clinical utility and BTM has been commercialised in the US, Australia and Europe with further expansion targeted in key markets including India & China.
Accelerating BTM sales addresses cash position
· Record revenues reported during 1H22 of A$18.2m (42% increase from 1H21) have been followed by accelerating BTM sales with record 3Q22 performance (A$12.3m).
· The cash position has improved from A$3.3m (as at 31 December 2021) to A$3.8m (as at 31 March 2022). This does not include proceeds from sale of land & buildings (A$6.4m).
· The strategy during 2H22 and FY23 will be to continue expanding sales personnel to engage key hospitals & clinicians and drive utilisation of NovoSorb BTM.
Key clinical trials and Polynovo pipeline
- Recruitment in the pivotal trial evaluating BTM application in full thickness burns commenced in 1H22.
- The target cohort size is 120 patients and data from this trial may be used in a potential label expansion to include full-thickness burns.
· The second arm of the SynPath study is underway and is examining BTM in the management of diabetic foot ulcers. This will assist in obtaining reimbursement in the outpatient setting.
Investment view: Transfer Coverage with Buy, Target $1.50
· We recommence coverage of Polynovo with a Buy recommendation and a 12 month price target of $1.50.
· The price target has been generated with a blend of two methodologies we apply to the company: EV/Revenue and DCF.
Yesterday (13/12/21) James Mickleboro from The Motley Fool shared a note out of Macquarie Group Ltd (ASX: MQG) on Polynovo in his article titled ‘Polynovo (ASX:PNV) share price tipped to double in value’ as follows:
“That note reveals that Macquarie has an outperform rating and $2.85 price target on the company’s shares.
Based on the current PolyNovo share price, this implies potential upside of greater than 100% for investors over the next 12 months.
Although Macquarie acknowledges that PolyNovo is underperforming its expectations so far in FY 2022, which has led to a sharp reduction in its earnings estimates, it remains positive on the future.
This is due to its belief that PolyNovo is well-positioned for growth over the medium to long term thanks to the NovoSorb product. Particularly as the company looks to expand its use into other areas such as the hernia repair and breast augmentation markets.
These are much larger opportunities than its current target market of dermal scaffolds (worth $1.5 billion per annum) and are estimated to be worth US$3 billion per annum each at present. Overall, this gives the company a $7.5 billion per annum market opportunity to grow into in the future if all goes to plan.”
The outperform rating by Macquarie along with the Polynovo’s record sales announcement this morning might have short sellers scrambling to close positions over the next few days.
Disc: shares held IRL & SM

I just don’t get it. After highlighting a pleasing Q1FY22 result in BTM sales, orders and accounts, Chairman David Williams goes on a bazaar tangent about the impact of shorting on the PNV share price. It was pretty clear from the graphs that the share price went in the opposite direction to the level of shorting. It also demonstrated that DW understood clearly the impact of short activity.
For a short time after the Q1FY22 announcement the short activity decreased and the price went up, which is what you would expect.
Then comes the bombshell on 5 November…The CEO/MD Resigns! If this is not bad enough the Board goes into inflammatory detail about the circumstances of the resignation, quoted below:
‘However, in more recent times there have been increasing differences with the Board in relation to Paul’s interaction with the company’s senior management team and his management style. Accordingly, the Board has accepted Paul’s resignation.’
This was like throwing petrol on the short activity fire! Why would the Board air it’s dirty laundry like this? Surely an expert on the impacts of short activity (DW) would have known what was to follow. DW has nearly 3% skin in the game, the most recent shares purchased on the market on 9 March 2021 at $2.34 per share. DW is hurting as much as anyone.
In 3 days from the 5 November to 8 November shorted stock increased from 6.19% to 6.84% (up 11%). Shortman.com lags ASX short activity by 4 days. I’m guessing short activity has spiked further since then, possibly over 7% by now. I don’t see this trend changing until there is some good news from PNV.
So what now? Polynovo is looking cheap on the fundamentals, current growth and future potential and I have continued to add shares to my IRL portfolio. But has the Golden Goose temporarily lost its mind? I guess we’ll need to wait for a new CEO to find out. Let’s hope it’s someone exceptional as I think this is what’s needed to gain some confidence in the management of Polynovo.
The AGM was a bit unusual in that the chairman David Williams ended his introduction with an interesting dissection of PNV share price movements in the past year. He attributes the sp volatility to short interest, which is currently quite high at 6% of issued share capital, the same as it was this time last year. After last year's very positive AGM, the short interest plummeted to 2%, creating a lot of buying pressure which led to a spike in the share price.
It seems very likely that this pattern will play out again after this year's AGM, which was also full of positive news, particularly about strong sales in the US in the past quarter. The only slightly negative sentiment from the meeting is that the company continues not to set any annual revenue target. The justification given for this is that the company is still early in its growth trajectory, and has not yet established a consistent, predictable pattern of growth.
This doesn't worry me in the slightest as a long term holder (in RL). There are so many avenues for growth - selling the existing BTM product into more markets, broadening the use cases for this product, then bringing on the new hernia product in 2023 - and the product is so superior to the competitors' products, that I'm struggling to see anything but a bright future. The big capital investment in manufacturing capacity is behind them, and now the company is focussing on sweating those assets, as the MD Paul Brennan stated.
FY 2021 AGM PNV
#Targetinggoldstandard #Morecountries #Morehospitals #Moreuses
Major take-aways from AGM:
Polynovo products entrance into new hospitals - up 56% YOY
FY22 Q1 $8.05 million (including patchy September with US lockdown)
New countries on board Q2 Q3 and Q4:
Cyprus
Italy
Czechoslovakia
Canada
France
Sales team now following up in hospitals across multiple wards:
1. Burns/amputations/plastics
2. Oncology
3. Endocrinology
Polynovo has taken 4 years to reach break-even (actually tiny profit)
Awaiting BARDA status in the US for full-thickness burns – this will be more for bragging rights as PNV will be able to officially declare itself as the Gold Standard for burn care (formerly held by Integra IDT biologic)
Increasing number of small size BTM product sales – better margins
Beta cell study in Type I diabetics about to launch
David Williams provided a good explanation about PNVs aim to enter US government stockpiles with their BTM product.
US government is looking to stockpile products for burns treatments, in case of incidents of mass injury. BTM can easily be stockpiled and has a shelf life of 3 years (stored below 25 degrees Celsius which is standard for medical products).
This is the other major advantage of this product over biologics which cannot be mass produced and stockpiled like BTM.
My Summary of PNV achievements, strategies and FY22 outlook
What does Polynovo (PNV) do?
PNV produces novel medical devices using patented bioabsorbable polymer technology Novosorb. PNV currently has 56 international patents covering their products. Its leading product is Novosorb BTM.
Novosorb BTM is used in the repair and treatment of burns and wounds and reconstruction. New products Syntrel BTM is currently undergoing studies for breast and hernia reconstruction and SynPath BTM line is used in chronic wound healing.
Novosorb BTM has been applied in a range of different demonstrated cases from new full thickness burns to old cases of scarring and regraft to wound care.
SUMMARY of why this companies product is so ground breaking (think cochlear for skin surgeries)
ADVANTAGES of Novosorb BTM – Synthetic resorbable polymer
Synthetic means man-made – unlike competitors that use animal products (e.g Aroa)
Resorbable means that the body can break it down to harmless by products and slowly excrete it (e.g through urine).
It is a temporary structural scaffold to allow the bodies’ own natural cells to regrow – BTM merely supports the body while is undergoes this regeneration and then the body removes the product.
Novosorb BTM has inbuilt design flexibility and biocompatibility
Key advantages include:
1. Reduced infection rates: The polymer product is not a food source for bacteria so BTM limits bacterial growth. It can remain in situ and any infections can be treated without product removal. Biological graft techniques are more prone to infection – bacteria spread through biological tissue more readily. This means large tissue sections have to be removed, the infection treated, then new grafts placed.
2. Minimizes contracture – this means shrinkage and wrinkling of post graft skin is reduced. Improves the cosmetic look and function of the skin. Contracture of skin can lead to immobile limbs that permanently stay bent as the skin is so tight it can’t stretch and fully straighten.
3. Can aid in generating new skin over bones and tendons – wound healing
4. Helps to salvage limbs as the new skin growth is vascularized (has blood flow) so can keep limbs functioning and avoid amputations
COVID IMPACTS
Polynovo (PNV) acknowledges the huge impact of COVID on its FY21 growth strategy in its latest report out to market today. What has impressed me as a Strawman and IRL shareholder is PNVs very quick pivot to online strategies for growth. This was primarily done in the form of online conferences to demonstrate advantages of their BTM product. Surgeons were able to gain direct online feedback from peers around the globe who had applied the product in real life cases.
Why I am happy with my investment:
Increasing Geographical Coverage for POLYNOVO products
European market
Growth for FY21 has been significant, despite COVID interrupting traditional marketing strategies.
PNV is predicting very strong growth to continue and new hospital accounts to increase during FY22
European breakdown
· Hard hit by COVID
· CE mark for BTM achieved just as Europe locked down- very challenging
· Sales team in EU couldn’t get in front of hospital decision makers and doctors in traditional meet and greet methods
· Moved to online meetings – PNV stated that they were able to have wider reach and took more online meetings than they would have taken with traditional on road approach
· During FY21 and FY22 Increasing sales headcount is a priority across UK EUROPE and IRELAND
· BTM now accepted into 16 countries as of time of update
UK/IRELAND
· Elective procedures cancelled during COVID
· Digital strategies only due to hospital closures
· Data published in key surgical journals
· BTM recently highlighted in hospital documentary
· Now only just being allowed to meet face to face
US market
Business rapidly expanding in revenue and sales personnel and continued growth expected in FY22
US breakdown
· 36 sales team members
· US based finance
· Market awareness and commercial growth increasing
· Best talent recruitment strategy adopted
· FY 21 US organisation has more than doubled
· New GPO agreements
· PNV intend to take market share through continued market penetration
· Weathered COVID well
· Built surgeon based webinars
· Despite challenges:
o 49% growth in sales FY21.
o FYH2 sales grew by 38% above and beyond FYH1 – indicating start of COVID recovery.
o FYQ4 21 pick up 40% more orders than in Q1
o Added 52 additional accounts in FY21
o More accounts in trial phase currently. These numbers are not added to reports until cash flow achieved.
· Acknowledge that Hospital closures really did affect sales
· Full US coverage planned
· Clinical and medical education planned as part of growth strategy
· BTM becoming standard of care at many hospitals in the US
Australian market
· COVID impact less is Australia
· FY21 2nd year following TGA approval in Australia
· Growth phase
· 2 new head counts
· 4 person sales team
Strategy for growth in following addressable markets in Australia
· Plastics and reconstructive surgeries
· Trauma in degloving and crush injuries
· Necrotising soft tissue infections
· Burn centres
· Wide excisional skin cancers
· Congenital disorders
· Chronic wounds (saving limbs from amputation) – targeting vascular surgeons
New Zealand market
· Burns centres comprehensively used Novosorb BTM
· Widely adopted by plastics and reconstruction teams
· Very successful market past 3 years and widely adopted
· White island volcano success stories
· FY21 and FY20 similar results
· Beat market sales expectations
Global growth
53% increase in global distributors sales growth in FY21
Australian growth
25% increase in Australian distributors sales growth in FY21
US growth
Up 49% in sales growth FY21
2020: US $10.4M
2021: US $15.5 M
Net profit after tax
Up 121.5%
2020: US $-1.2M
2021: US $0.2M
EBITDA (Achieved break-even / profit in FY21)
UP 161.1%
2020: US $-1.0 M
2021: US $ 0.6 M
Cash on hand
US $7.69M
BARDA Trials
Pivotal Burn Trial full completion FY25 and Chronic Wound Reimbursement Trial Diabetic Foot Ulcers (completed first 10 patients and moving on to trial phase 2) continue in FY22
Chronic Wound TAM
SynPath chronic wound product has predicted US $400 M market in FY23
PNV is investing in itself and planning for growth
New Facility and upgrade: Port Melbourne
· Tripled current manufacturing footprint
· Installed new ISO 7 clean rooms for the production of medical devices
· Increased storage areas – aim to store and distribute from facility (some devices are temperature sensitive)
· Duplicates BTM production sites – allows for increased demand and built in redundancy to sure up integrity of POLYNOVO supply chain
· New equipment already installed to support Syntrel Hernia product line
· Initial Syntrel Hernia production runs have been done – product in testing phase
· New Equipment will allow for Bioresorbable Polymers to be produced in a range of different formats for multiple indications – what would take months, new equipment can produce new product line in weeks or even days to get product to market immediately.
· All products now completely produced in-house
Future TAM and product line potential is huge.
PNV has become a multiple trick pony.
Future Applications for PNV
Beta-cell diabetes application joint collaboration with Beta Cell Technologies Pty Ltd.
Using Novasorb BTM in alternative application forms. The plan is to infuse BTM with Pancreatic Islets in the skin for diabetes treatment.
A trial is planned FY22 for applying this new technology to Type I diabetics who have had renal transplants.
Novosorb BTM has potential as a drug elution delivery system.
Ongoing exploration of new addressable markets and applications for this product. New facility in Melbourne will allow light-speed alteration and development of products, mass productions and delivery to market.
Added Strategy as ESG responsible investment
PNV aligned with ESG focused responsible ETF investments. Potential for SP growth with adoption of PNV into larger fund portfolios.
Aiming for carbon neutral certification by FY23
This mostly repeats Shrivak's straw, but these are my takeaways from the investor presentation and call.
Turned a profit for the first time (if you exclude share-based payments)
49% revenue growth in US to $15.5m, despite Covid , still ramping up sales team,which grew from 18 to 36. This region is profitable and the largest revenue contributor.
25% revenue growth in Australia
Total revenue growth 32% to $29.3m
Entered 8 new jurisdictions in Europe and Asia via distributors
Revenues will increase faster than costs from here on, capex costs were down 60% after commissioning of new factory
Cash on hand $7.7m - no change from 6 months ago
Growth is all about increasing the sales force to get in front of more surgeons
Manfacturing capability for hernia devices is complete, animal trials in progress, working toward FDA approval in March 2023
This is a fabulous Australian innovation story, with a world-beating product and a clear growth path through geographical expansion and development of more products based on the BTM technology to address more use cases. Covid has been a very significant headwind this year since face-to-face selling (to surgeons) is the primary means of acquiring new customers, so to achieve 32% revenue growth in these circumstances is pretty impressive.
former hot stock, sales down in pandemic due to less people out and about getting burns injuries.
38% growth 2H on 1H
small profit
Yesterday the PolyNovo share price plunged around 9% closing at $2.10. James Mickleboro from Motley Fool said "the decline appears to have been driven by a couple of broker notes this morning in response to its sales update on the 13 July. Both Bell Potter and Ord Minnett have downgraded the medical device company’s shares to hold ratings and cut their price targets. Bell Potter’s price target has reduced to $2.65 whereas Ord Minnett has cut its price target to $2.54."
I think the market is over reacting to the re-rate. Bell Potter cut their price target buy only 10c/share from their previous target of $2.75 and recommended HOLD. The market seemed to have interpreted this as sell, and sell quickly!
I note the new target price is similar to the Strawman consensus valuation. It is hard to value PNV right now, but I think it will grow rapidly when the COVID impacts decline, more hospital beds become available, and the sales team can travel around and do their job. One analyst on Simply Wall Street thinks earnings growth will be 78% over the next 3 years (see chart below). I think the stock is now a buy and is undervalued by at least 30%.
Disc: Adding to RL portfolio


