Consensus community valuation
Straws are discrete research notes that relate to a particular aspect of the company. Grouped under #hashtags, they are ranked by votes.
A good Straw offers a clear and concise perspective on the company and its prospects.
Please visit the forums tab for general discussion.
Here's the announcement on the FDA engagements regarding HIE and PTHS clinical development for NNZ-2591.
Basically, Neuren has received written FDA feedback (not a meeting as requested) confirming a clear regulatory path for NNZ-2591 in both HIE and Pitt Hopkins syndrome.
Additional juvenile animal data is required before initiating the HIE IND, i.e., in human Phase 1 clincal trial. So, a minor delay.
And there is alignment that a PTHS-specific CGI plus a functional observer-reported endpoint could support a controlled efficacy trial. Here, I think $NEU are being customarily cautious not to refer to the terms Phase 2 or Phase 3, because we haven't yet heard the readout from the Phase 2 submission currently under consideration. Obviously, $NEU would hope to move into a pivotal Phase 3 trial, but by not using that term now, they avoid a potential future disappointment. Well, that's my reading of it.
Neuren plans to advance both programs in 2026, while continuing its Phase 3 PMS trial, with no material funding impact from the FDA feedback.
I don't consider that this announcement moves the needle one way or the other.
What will in my view move the needle, is any positive update on the ongoing PMS Phase 3 trial. However, as it is a randomised, double-blind, control trial, I think any update will relate to information about progress in enrolment, absence of serious adverse events (maybe), and any update on the foward timeline.
In summary, I don't see a lot here today. Do others have a different view?
Disc: Not held
Feb-26
I was going to await the latest DAYBUE results from $ACAD (expected 1-March) before updating, but today's adverse EU news, moves my current view of value even further away from my previous SM valuation. So I will update my valuation as a placeholder to take accout of both 1) last year's data on US sales profile for DAYBUE and 2) today's news from EU, which adds to the Canada "do not reimburse" recommendation last year.
Placeholder: DAYBUE reduce to $10, add 50% to the p50 for NNZ-2591 gives $8 for a sum of parts valuation of $18, but with wide uncertainty.
Uncertainty is very wide. For example, in the downside case DAYBUE could be worth <$10 depending on longer term US persistency and ultimate market penetration. And, in the failure case, NNZ-2591 could be $0.
Feb-25
No change to valuation $24 ($12-$46). "Two-for-the-price-of-one" thesis intact.
Good progress on DAYBUE has probably reduced the likelihood of the downside cases, however, I will wait for clarity on the likely European trajectory before updating from the DAYBUE perspective, assume US hit guidance (so at least 12 months for that).
Aug-2024
See Valuation Straw $12-$46 based on acquisition multiples.
Wide range due to including NNZ-2591, which is still at Phase 2.
Sum of Parts
DAYBUE: $12 - $16 - $20
NNZ-2591: $0 - $16 - $32
SUM: Pick a number! Take p50 DAYBUE + 50% p50 NNZ-2591 ... to more heavily discount the development risk = $16 + $8 = $24.
I know its a bit sloppy, but hey, look at the assumptions I've made!
-----------------------------------------
Dec-2023 $31.00
Today's news on NNZ-2591 materially impacts the value of $NEU.
While I have not modelled NNZ-2591 explicitly, I have tweaked my existing DCF scenarios, which are based on 2 factors: $NEU revenue growth (x4 scenarios) and ultimate penetration of the Rett's market (x2 scenarios) - so 8 scenarios together.
Two sets of scenarios assume NNZ-2591 fails, and that $NEU revenues start to decline from 2029 and 2033. Given the now significantly increased likelyhood of NNZ-2591 leading to a second and even more material revenue stream, I have reduced the probability of these scenarios by half, from 35% to 17.5%.
I have also removed a valuation discount allowing for the emergence of a competitor to DAYBUE. The rationale being that NNZ-2591 success will start to drive revenue from 2026 in the success case, which is earlier than my model (2029).
After these updates, keeping everything else unchanged, the [p10, p50, p90] estimates of value are: [$16, $32, $50], as seen from the plot below.
I will do a more complete model rebuild when we have the Full Year result for DAYBUE and hopefully an update on the NNZ-2591 timeline, in February 2024.
(None of the scenarios consider the extreme upside case that NNZ-2591 ends up being approved for all four conditions. We have to seriously consider that, because the positive animal model effects seen in PMS have carried through to the Phase 2 human trials. There are positive effects in the other animal models - what if these translate? We're then looking at a completely different story. But, too early to evaluate this.)
Basically, with the SP today at around $22, I see a compelling risk-reward upside from here.
Disc: Held in RL (6.2%) and SM

A blow today for the international rollout of trofinetide by $NEU's US partner $ACAD after a "negative trend vote" by the EU's CHMP on the MAA.
$NEU SP is down around 8% at time of writing, bouncing off an initial low of -15%.
What is the significance of a "negative trend vote"?
Basically, CHMP gives MAA applicants an indication of the direction of travel in its consideration of MAAs (drug approval applications).
A peer-reviewed study conducted over a decade ago showed that a negative "trend vote" results in 75% of applications ultimately being declined, so the news is not good.
$ACAD appears likely to request a "re-examination", however, the odds of success are not strong. Evidence indicates that when requests for re-examination are successful, any subsequent approval is likely to involve label restrictions, wherein the drug is approved for a narrower use case.
From my understanding of Rett, it is hard to imagine what the limitation might look like in this case. Perhaps an age restriction or possibly in cases with the most severe limitations,... but I am grasping at straws here.
As I commented on and predicted last year, the Canadian technical committee "do not reimburse" recommendation after approval in that market looks like it was indeed the canary in the coalmine. It gave me sufficient pause for thought, and weighed heavily enough on my valuation as I assessed it to add a lot of risk to the global rollout of trofinetide, so I sold my entire holding at that time.
I consider that management at $ACAD and $NEU did not communicate clearly enough the Canadian decision to investors, and in the case of $ACAD it was almost swept under the rug, coming to my attention only as a Q&A item at an industry conference presentation!
This underscores for me the importance of taking the time to attend every conference and getting hold of every transcript. Pharma and Medtech is complex, and while each market makes it own determination, decisions in one jurisdiction should be considered in assessing the chances of success in another.
Sadly, for me, this further undermines my "two-for-the-price-of-one" thesis for $NEU, wherein the value of business is covered by DAYBUE, with NNZ2591 being a potentially very material upside.
I'll reconsider my valuation for $NEU once I have the next quarterly result for DAYBUE from $ACAD - my current view being that the product in the US is tracking to a lower peak sales outcome that in my more optimistic scenarios.
So I am watching, and no long hold $NEU, and am less confident that I will hold again, unless there is a significant SP decline so that the risked value of NNZ-2591 becomes compelling. That would potentially require the SP to fall below $12 and, even then, there is probably no hurry given the CT timelines.
Disc: Not held
I just listened to the JP Morgan healthcare conference that Acadia presented at and it is pretty positive for Neuren. The surprise was a longer dated sales target of $700m by 2028 for Daybue. This has some pretty significant flow on effects for NEU if it is achieved in terms of milestone payments.

Three scenarios -
1) sales increase in the US and they manage to exceed the $500m threshold for the next $50m payment. I think this is likely as the 2000 patients that have tried Daybue of a total US population estimate of 6000 potentials (up 10% from previous estimate). So probably somewhere around 3-4000 actual patient pool. No mention of Canada approval solutions, so probably not likely to contribute anything by 2028. They mentioned the investment in sales people was having a positive effect. But to me it seems unlikely that they will get to $>750m milestone ($100m payment) from the US alone so I think this will be the final milestone payment from the US for a while and maybe forever. So then they would transition to a steady $60m or so per year of sale royalties
2) A big chunk of these sales come from outside the US, as they expect Europe approval Q1 2026 and an extra phase 3 trial for Japan will be completed in 2026. They were fairly positive on both of these. They will get $35 and $15m milestone payment for first commercial sale, which looks very likely. Then the sales milestone targets are not defined for the ROW, but are up to $170m for Europe and $110m for Japan. I am thinking the initial trigger levels of these payments will be higher than in the USA as they are getting a higher sales royalty - high teens to low twenties vs low teens in US. So I am expecting them to hit some milestone payments but what they are is largely unknown beyond its moving in the right direction.Another unkown is the actual pricing for these jurisdictions haven't been determined as far as I can tell, and this could impacted the sales quantum.
3) Have overpromised, and the new STIX formulation doesn't address the problems that some patients have with the oral liquid, so potential patient pool is still limited to those who tolerate it or perceive it to work. I can't assess this but from what they say about STIX it sounds like a good option, it can be mixed with any liquid (apple juice to gatorade) so should be more palatable, I still shudder at the thought of the unpleasant cherry flavored medicine from when i was a kid. STIX is lower in carbohydrates, which is impoartant as many of the patients follow a KETO diet and limit sugar intake. STIX is also more convenient- doesn't need to be refrigerated, much easier to travel with etc. Overall it does sound like it could be an accelerator but will watch and see. So from what I have seen it looks like a product improvement, they will start selling in Q1 but wont ramp up to full availability til Q2, they also said they will sell each version, but its hard to see why anyone would want the oral solution given the benefits of the STIX formulation.
Its likely to be some combination of these scenarios but I still like NEU, the ability to get a steady income while your developing a pretty exciting drug in NNZ2591. Happy to keep holding this one.

There are a number of positives in this announcement. Firstly, the 700m sales (admittedly in 2028) is higher than expected. Second, the persistency rate increasing is obviously great (and presumably feeds into the higher sales projection). And thirdly, the timeline for results in the Japanese trial is reassuringly early.
SP up over 6% so far today
Today NEU announced:
“Melbourne, Australia: Neuren Pharmaceuticals (ASX: NEU) today received notification from the US Food and Drug Administration (FDA) of an administrative delay to the scheduled pre-IND meeting for NNZ 2591 in hypoxic ischemic encephalopathy (HIE), which is now expected to occur in late January 2026 rather than in December 2025.”
The change of FDA administration (Robert Kennedy & Co) and the staff cuts of around 20% have been well telegraphed. Even if you subscribe to the view that the FDA was loaded with bureaucrats and loafers, it is hard to believe you can take out 20% of the staff and it will still function normally. Add to this trouble the recent Government shutdowns.
The FDA is hyper politicised, and you wonder just what pull the likes of small fry like Neuren, Clarity Pharmaceuticals (ASX:CU6) or PYC Therapeutics (ASX:PYC) would have with the FDA compared to the likes of a Merk or Eli Lilley as they fight it out for FDA time slots. Overseas biotechs may face an underlying bias of the current administration around prioritising American business. If things are getting tighter at the FDA it is likely bad news for Australian biotech.
NEU with net cash of $300m and ongoing royalties from Daybue of around $100m/yr is likely to be able to weather this situation better than most.
But for a CU6 that is tearing through around $90m a year, and even with $190m currently in the bank, say a 6 month delay to the lead product caused by FDA delays would be very damaging . That bag of cash is suppose to get CU6’s lead diagnostic to commercialisation. PYC is another high stakes biotech with cash flooding out the door and sweating on hitting clinical and FDA milestones.
These biotechs like to rabbit on about FDA “Accelerated Approvals”, “Fast Tracks”, Breakthrough Therapy” and “Priority Reviews”. However if the FDA is not functioning like it should there is FA they can do about it.
With the "Canada issue" out of my system in Part 1, here is a summary of last week's $ACAD (Acadial Pharmaceuticals) 3Q Results call.
1. Commercial Performance and Market Dynamics
- Net sales: US $101.1 million in Q3 2025, up 11% year-over-year, marking Acadia’s largest quarterly sales for DAYBUE. This was the highest quarter for net product sales overall
- Market penetration: Approximately 40% overall in the U.S. Rett syndrome market, with 27% penetration in community settings
- Referral growth: Achieved the largest quarter-over-quarter increase in referrals since launch, driven by expansion of the field team and outreach to community prescribers.
- Community prescribers: Now account for 74% of new prescriptions, up from 64% the prior year.
- Persistency: > 50% of patients remain on therapy at 12 months, and > 45% remain on therapy at 18 months, indicating durable adherence.
- Global access: Named-patient supply programs are expanding in Europe, Israel, the Middle East, and Latin America, with 1,006 patients treated worldwide to-date
- Guidance (FY 2025): DAYBUE net sales forecast US$385 – $400 million, with gross-to-net 22.5 – 23.5%, reaffirming momentum
(From Q&A)
- Management reiterated that growth was broad-based across both academic and community prescribers, with community uptake now sustaining most new patient starts. (Note: some 75% of the addressible market is treated by community prescribers, and this segment remains relatively under-penetrated)
- They emphasized that the larger field force deployed earlier in 2025 directly contributed to improved referral rates.
- No change was announced to 2025 guidance, indicating confidence in continued double-digit growth.
2. Regulatory and Geographic Expansion
From presentation
- DAYBUE (trofinetide) is approved only in the U.S. and Canada for treatment of Rett syndrome in adults and pediatric patients ≥ 2 years
- A Phase 3 trial of trofinetide in Japan has been initiated, marking Acadia’s first pivotal trial outside North America
- The company filed a Marketing Authorisation Application (MAA) with the European Medicines Agency (EMA), with an anticipated CHMP opinion in 2026
- Named-patient supply programs already operate in multiple regions (EU, Israel, Middle East, Latin America).
(From Q&A)
- Executives stated that the EMA filing was accepted in Q3, and the CHMP opinion is expected mid-2026.
- They confirmed that Japan’s Phase 3 trial uses the same dosing and endpoints as the U.S. approval study, designed for regulatory alignment.
- Early named-patient use is providing real-world data that will support broader access discussions post-CHMP.
- "Disappointing decision in Canada" (what??! See Part 1 Straw)
3. R&D and Pipeline Integration
From presentation
- DAYBUE remains the core commercial rare-disease asset, while ACP-2591 (cyclo-GPE analogue) is a next-generation follow-up in Rett and Fragile X syndromes
- The company is building a global Rett-syndrome franchise, leveraging DAYBUE’s success to expand to additional indications and geographies.
(Q&A)
- R&D leadership highlighted that DAYBUE’s durable efficacy data continue to inform the design of ACP-2591 Phase 2 planning, with a goal of complementary rather than cannibalistic positioning in Rett syndrome.
- Management also noted interest from academic consortia in exploring DAYBUE in combination therapies, though no new trials have yet been initiated.
4. Other Q&A Comments
- CFO Mark Schneyer confirmed that DAYBUE’s gross-to-net deduction (~23%) is expected to remain stable in 2026 given payer mix and limited rebate pressure.
- Analysts asked about inventory levels; management stated they are “healthy and in line with volume growth,” with no channel stuffing anticipated year-end.
My Assessement (thinking out loud, so apologies for the stream of consciousness format)
DAYBUE sales of US$101.1m for the Q came in below the low case of my model ($102.3 - $104.3 - $106.2). My model had them hitting a FY range of $388.5 - $400.5, and so with the lower number achieved in Q3, I'm expecting they'll be towards the lower end of the narrowed guidance of $385 - $400m.
But this is really splitting hairs, and the key message is that management have confirmed my view form earlier this year that they have a good grip on how the product is performing in the market, and sales force productivity, and so will be making good resource allocation decisions.
What we don't know is how much of the revenues came from outside the US, given that the product is being made available to patients ex-US in an increasing number of markets in early access programs. It's a high-value product, so it doesn't take for many patients accessing the drug from overseas to start to move this dial.
But I am disappointed at the growth rate, because in Jan-25 the company announced a 30% sales force expansion. This was completed in May-25, and while it will have taken some months for new starters to get traction with their accounts, the period Jul-Oct has in my view enjoyed a full quarter with the expanded field force, focused on the community-based prescribers. My range was meant to capture the full ("80%") confidence I perceived at the time.
A year ago, the 3Q-on-2Q growth rate was +7.8%, so even with the +30% expanded sales force in 2025, the sequential q-o-q growth of +5.2% indicates just how much of a headwind patient churn is. That said, Persistency seems to be holding up: c. 50% at 12 months and 45% at 18 months are again reported, and it will probably not be until next year that we get to see what this is becoming out to 24 months.
So, let's have a stab at considering US Revenue Growth from here:
Case 1: Management say they will continue double digit annual revenue growth. So giving them the full benefit of the doubt and assuming they hit 2025 revenue of $390m, at 10% for two years, that gets them to $472m in 2027, and $520m in 2028 - likely the year of plateau sales,... or maybe they'll eek out some further growth in 2029.
Case 2: Being generous, let's assume an upside QoQ growth of 4% or 17% pa for two years. That gets them $533m in 2027, and a likely plateau sales in year 5 of $623m in 2028.
But, then again, I think Case 2 is overly generous. Afterall, the 2025-2024 PCP growth rate was only 11%, and let's trim that to 10% to allow for some overseas patients. The sales force expansion has helped the last quarterly result, and so they will likely struggle to do much better than 10% p.a. over two years, even if the 2026 FY number gives a full year with the expanded salesforce cycling an earlier year before the ramp up, and so exceeding 10% in 2026 should be doable in all scenarios. But it can't be sustained IMHO. The persistency drag is just too high.
Looking at the historical trends, the rate of growth is maturing rapidly. For example, even while benefiting from a full quarter of expanded sales force, 3Q/2Q-2025 was +5.2%, compared with 3Q/2Q-2024 at +7.8%. So there is defintely a sequential decline at play towards a plateau that doesn't look that far off (s-curve).
So, in the table below I've modelled three scenarios for annual US revenue growth over the next 3 years (all in $USm):
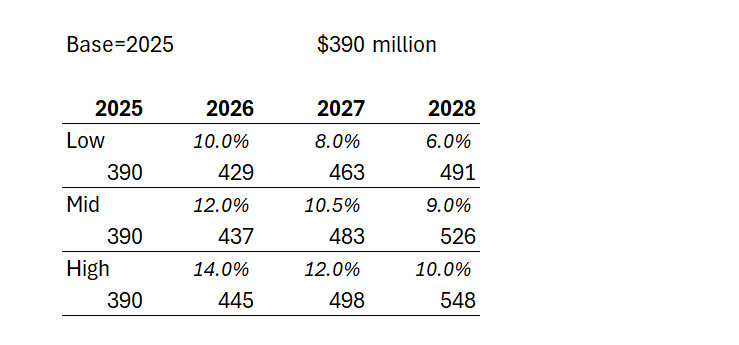
Depending on how longer term persistency evolves, we could find either 2028 or 2029 as the plateau year for the US. And in the illustration above, in the low scenario, $NEU doesn't get anymore milestone payments, and on the other two scenarios, will get the US$50m milestone only in 2028. The $750m milestone looks well and truly off the table to me.
I haven't pulled this through my $NEU valuation model yet; however, it is a significant deterioration on my earlier projections.
So, what does this mean for $NEU?
Well, US sales of US$490-$550m in round numbers are $NEU royalties of US$54-$62m or A$83-$95m, and that's much, much lower that the analysts have for $NEU.
Why?
Because I think the analysts are including all of the following things I haven't included:
- Assume continuing growth, and not modelled an s-curve with a continuing persistency driving decline after year-5 plateau
- Added milestone payments
- Added RoW
My model is too complicated to pull through a simple set of number for the value of DAYBUE to $NEU. However, my earlier projections gave me $12 - $20/share (US growth and pro rata RoW offset by 3-4 years)
Today, I believe US DAYBUE will have a plateau in 2028 or 2029 and immediate steady decline, as new patients arrivals fail to keep pace with churn. So, if all we have is the US, then valuing $NEU at 5x peak contining royalties of, let's be generous and say, A$85m to A$120m gives A$425m - $600m, or with SOI of 126.5m shares, that's $3.35 - $4.75 /share.
Yes, that's what $NEU (US-DAYBUE only) could be, if it can't get reimbursement beyond the US and if it can't sustain growth,
Wow.
Of course, I think it will be worth more than that. But hopefully, you can see that at $18/share, NNZ-2591 is now potentially doing a lot of heavy lifting. And it might be worth it. But it also might not.
But that's not what my "2 for the price of 1" investment thesis was all about. So my thesis is well and truly broken.
BIG CAVEAT: I wasn't going to post this, because I was sure I've made an error somewhere. But I have gone through everything a couple of times, and also used a couple of independent methods, and keep getting similar results. And to be clear, I haven't accounted for $NEU's massive cash pile. But I am not doing an enterprise valuation of $NEU, but rather only the value of the royalty stream from US DAYBUE. So, as ever, this is not advice. But it is enough for me to take the decision I have already executed. Oftentimes, more analysis doesn't add more value.
That said, over the coming months, I will properly update my model. And that's because I want to know at what price I am prepared to buy back in for the value of NNZ-2591. But I don't think there is any hurry. The PMS Phase 3 trial has some way to run.
Disc. Not held
I am in the process of writing up my notes from last week's Acadia Pharmaceuticals ($ACAD) earnings call, however, one of the most important insights in the call was a reference by CEO Catherine Owen to a question from Jack Allen (Robert W Bird & Co. Incorporated) about reimbursement in Canada.
TLDR: The Canadian Drug Expert Committee of the CDA in August 2025 filed its decision: "Do Not Reimburse" for trofinetide (DAYBUE)"
I've decided to elevate this to a stand-alone straw for two reasons. First, it is bad news, and in my opinion it could have a significant bearing on the pending EU/UK approval outcome (even though these are completely independent of each other).
Second, I am concerned that it demonstrates a lack of candour by both Acadia and Neuren management, given that I have conducted an extensive search and can find no proactive references to it in investor communications. (And of course, if it is proven I have missed something, then I withdraw this comment unreservedly. This is also why I am flagging the point, in case other eagle-eyed $NEU investors have picked up something I've missed.)
On the call, analyst Allen asked: "I wanted to ask about reimbursement in Europe. I know there were [... inaudible,.. line cut out] ... in Canada over the summer...."
CEO Catherine Owen replied; "Thanks, Jack. So yes, we are obviously in the middle of discussions and thinking right now around reimbursement in Europe. And you're right, we did have a disappointing decision in Canada. Tom, do you want to share a little bit more about how we're thinking about reimbursement in terms of the sequential approach to that in Europe?"
Tom Garner (VP Commercial) continued ... absolutely no reference to Canada!
Both companies have proactively disclosed and continue to communicate that DAYBUE is approved for use in Canada. And that is true. But after approval (based principally on efficacy and safety), a drug has to gain a reimbursement decision. For countries with a mixed public and private healthcare system, the reimbursement outcome by the responsible public agency, has a significant bearing on prices that will be covered by entities like health insurance payers. So the public reimbursement milestone is critical for determining the revenues that can ultimately be achieved in a market.
And yet, the only reference to the Canadian issue either verbally or in writing that I can find are the following words by Catherine Owen "we did have a disappointing decision in Canada". (Again, other $NEU shareholders, please tell me what I've missed.)
So, I've structured this straw as follows:
1. The Canadian CDA-CDEC Decision
2. How Reimbursement Works in Canada
3. Why I think there is a potential, broad "Read Across" to other Jurisdictions
4. Implications for Valuation
5. Investment Decision
----------------------
1. The CDA-CDEC Decision
I attach the link to the full CDA-CDEC decisions. These are published for transparency in the Canadian Journal Of Health Technologies. It makes for interesting reading.
My BA has summarised the document for you as follows:
Summary of Decision
Agency: Canada’s Drug Agency (CDA-AMC)
Committee: Canadian Drug Expert Committee (CDEC)
Date: August 2025
Decision: DDO NOT REIMBURSE trofinetide (DAYBUE) for the treatment of Rett syndrome in adults and children ≥ 2 years of age and ≥ 9 kg.
Key Reasons for the “Do Not Reimburse” Decision
a. Uncertain Clinical Meaningfulness
- The pivotal LAVENDER trial (N = 187) showed statistically significant improvements in caregiver-reported behaviour (RSBQ –3.1 points, 95% CI –5.7 to –0.6) and clinician-rated global improvement (CGI-I –0.3 points, 95% CI –0.5 to –0.1).
- However, no minimal important differences (MIDs) were established, so the committee could not determine if these changes were clinically meaningful
- The trial had high discontinuation (≈25%), missing data, and relied on outcomes not used in Canadian clinical practice.
b. Lack of Evidence on Quality of Life and Function
- The trial provided no measure of health-related quality of life (HRQoL) for patients or caregivers, and data on communication, motor skills, and caregiver burden were limited and unvalidated.
- CDEC therefore could not determine if trofinetide addresses the unmet needs identified by patients and caregivers.
c. Very Low Certainty of Evidence
- The committee rated the overall certainty of evidence as “very low” due to bias, imprecision, and indirectness between the trial population and the broader Health Canada indication.
- Open-label extension (LILAC/LILAC-2) and supportive studies (DAFFODIL, LOTUS) did not resolve these uncertainties.
d. High Cost
- Annual treatment cost estimated at CA $427,000 – $1.34 million per patient, depending on weight
e. Adverse Effects
- Diarrhea (81%) and vomiting (27%) were common, leading to 17% discontinuations in the treatment arm vs 2% with placebo.
- While manageable, these events raised concerns about unblinding and trial validity.
f. Reconsideration and Patient Input
- Sponsor Request: Acadia requested reconsideration, arguing the RSBQ endpoint was valid and that CDEC had undervalued caregiver-reported benefits.
- Patient & Clinician Feedback: Groups emphasized the severe unmet need and the importance of even small improvements in communication and daily function.
- CDEC Outcome: Acknowledged these perspectives but upheld the original decision, concluding the evidence was too uncertain to demonstrate meaningful benefit or long-term safety
2. How Reimbursement Works in Canada
Now it is important to recognise that the report is a recommendation, and not a binding decision. However, for all practical purposes, I consider reimbursement unlikely, unless $ACAD can submit further evidence that addresses the grounds for denial. Clearly, the company will be working on this, and has in fact already had one shot at it.
Let me explain why I feel this is the case, by explaining how the system works in Canada.
The CDA-AMC conducts independent clinical and economic evaluations of new drugs and medical technologies on behalf of Canada’s publicly funded health systems (federal, provincial, and territorial, except Quebec). It does not set drug prices or directly fund products, but rather provides evidence-based recommendations to help governments decide whether to include drugs on their public formularies (lists of reimbursible medicines).
The Canadian Drug Expert Committee (CDEC) is an independent panel within CDA-AMC composed of physicians, pharmacists, health economists, and patient representatives. It reviews clinical trial data, real-world evidence, cost-effectiveness analyses, and patient submissions. The committee then issues a non-binding recommendation to federal, provincial, and territorial drug plans.
Provincial/Territorial Decision-Making: Each province/territory makes its own final reimbursement decision, typically following the CDEC recommendation. In practice, a “Do Not Reimburse” recommendation from CDA-AMC almost always results in non-listing (no public coverage) across the provincial drug plans.
Private insurers may make separate decisions but usually reference the same HTA findings.
Reconsideration and Transparency: Manufacturers can request reconsideration (as Acadia did for DAYBUE). The CDA-AMC publishes both the original recommendation and any reconsideration outcome on its website and in the Canadian Journal of Health Technologies for public transparency.
3. Why I think there is a potential broad "Read Across" to other Approvals
My reading of this situation starts to answer a question I always had on my "issues" list. DAYBUE is by any measure an expensive drug, and it was a question for me how jurisdictions would deal with it in places where cost-benefit analysis has a stronger bearing on the reimbursement decision. While the US is increasingly alert to healthcare economics, there is no question that it is more permissive of reimbursing high cost drugs where the cost-benefit is open to challenge.
It seems the Canadians have issues with both the strength of the effiicacy data, the side effects, tolerability and low persistance of the drug (all indications impacting Quality of Life) and the high cost.
The EU process will follow a similar path to Canada. The EMA-CHMP will make its decision on scientific grounds, whereupon each countries HTA (Health Technology Assessment) process will kick in, leading to the reimbursement decions. In the EU/UK, public medicine is a much larger share of the drug budget than North America, so the single-buyer country agencies will have a lot of clout in the pricing decision. And European countries have even greater pressures on public health budgets, given weak economies, unfavourable demographics, and a greater taxpayer contribution to the healthcare spend.
All European HTAs consider cost-effectiveness, clinical value, budget impact, and comparative benefit (albeit there is no other treatment for Rett). And so I believe the Canadian decision is a potential canary in the coalmine for Europe. Even though the European process is completely independent, I imagine all the European decision-makers will read the Canadian document. In Europe, there is also greater political pressure than in the US for challenging high cost drugs, and even more so when there are clear question marks on quality of life benefits.
In the best case scenario, I anticipate this will result in European prices heavily discounted to the US price. By heavily discounted, I mean 40%, 50% and even up to 70% or more!
4. Implications for the Valuation of Neuren

The Canadian "canary in the coalmine" puts a questionmark over the remaining value in the right-hand slide above. While RoW paitents may be given access to the drug on special grounds ("early access programs"), these are unlikely to trigger the "commercial sale" milestone payments. Privately-funded patients might also find ways to get access to the drugs.
The Canadian decision also indicates to me that negotiations towards an acceptable reimbursement decision could be protracted. $ACAD needs to hold out for a good enough price that make sense, given that it is on the hook for the initial milestone payments irrespective of the value of the commercial sales. So, in Europe, it will have to see revenues that cover: US$35m royalty + "mid teens to low-20's" tiered royalties + distributors margin (or fixed cost of sales force, if direct).
So with potentially a much lower price, a relatively high fixed royalty, and a lower Gross-To-Net, $ACAD will need to see material revenues, before agreeing to launch.
The reimbursement negotiation is therefore likely to be protracted. $ACAD will marshall all the real world data that they can from the US, as well as the continuing open lable trial data on long term persistency (now down to 45% at 18 months, down from 50% at 12 months) potentially pushing RoW cash flows further into the future.
5. Investment Decision
I haven't crunched this all in my model. However, on a risked view, I am materially marking down my Outside North America DAYBUE revenues (start, ramp, peak), and therefore also the royalties to $NEU (note: the percentage tiered royaties here are significantly higher than the US.)
By eyeballing my current model my "2 for the price of 1 thesis" is blown for $NEU at $18.
In round numbers, my valuation of DAYBUE ($12-$20), has clearly fallen to the lower end, with a significantly lower downside case if DAYBUE cannot achieve significant commercial traction outside the US.
Which means that much more of the current share price rests on the development of NNZ-2591. I had given NNZ-2591 $8 of value at a 50% risking (from $16). Perhaps that is harsh, but until I have a better view, then that is my number.
Which means that at a SP of $18, I need to see $10 of value in DAYBUE, and I don't see that with confidence, as I will make clear in my next write-up on the $ACAD results.
For me, the bottom line here is that more of the value in this business lies in drug development risk, rather than commercial execution. Looking across my entire portfolio, I am carrying quite a bit of this risk, and I am not confident that I have a good grip on the outlook for $NEU. I'd therefore like to take 2-3 quarters to evaluate DAYBUE's progress from the sidelines. And that's because I think there is a reasonable chance that we will soon have visibility of US DAYBUE plateau sales below US$500m (downside) and not much more (upside). I'll sketch this out in more detail in my next note.
Accordingly, I have sold my shares in $NEU.
Disc: Not held
Just wondering if anyone (@mikebrisy @Nnyck777 @BendigoInvesto) had any comment on their latest update?
Thanks
C
Record DAYBUE™ net sales of US$101.1 million in Q3 2025, up 11%
from Q3 2024
Q3 Highlights:
from Q2 2025
• Q3 2025 DAYBUE™ (trofinetide) net sales of US$101.1 million, up 11% from Q3 2024 and up 5%
• For the first time more than 1,000 patients received shipments
• Largest quarter-over-quarter increase in referrals since launch driven by expanded team
• 74% of prescriptions to new patients were written by physicians in the community setting outside
centers of excellence
• Named patient supply programs active across multiple regions outside North America
• Neuren earned Q3 2025 royalty income of A$16.4 million, up 24% from Q3 2024 and up 12% from
Q2 2025
• Acadia narrowed full year 2025 DAYBUE US net sales guidance to US$385 – 400 million (previously
US$380 - 405 million), implying full year 2025 US royalty income for Neuren of A$63 – 66 million
(previously A$62 - 67 million)
Another step forwards for Neuren on their journey to address new syndromes

$NEU have announced that their first site for the phase 3 trial of NNZ-2591 in Phelan-McDermid Syndrome (PMS) is underway.
Here's the short text:
"Neuren Pharmaceuticals (ASX: NEU) today announced initiation of the first investigational site in the United States for its Phase 3 clinical trial of NNZ-2591 in Phelan-McDermid syndrome (PMS), after receiving Institutional Review Board (IRB) approval. Other trial sites in the US are at various stages of the initiation process. This is the first ever Phase 3 trial in PMS, a serious neurodevelopmental disorder with no approved treatments. The randomised, double-blind, placebo-controlled trial will assess treatment for 13 weeks in approximately 160 children aged 3-12 with PMS. All participants may be eligible to continue treatment with NNZ-2591 for 12 months in an open-label extension trial. The trial program is fully funded by Neuren’s existing cash reserves. Neuren conducted an End of Phase 2 Type B meeting and a subsequent Type C meeting with the US Food and Drug Administration, at which alignment was reached on the design of the Phase 3 trial, including the primary efficacy endpoints."
My comments
So this is the Phase 3 trial for the first of several rareneurological conditions for NNZ-2951, and likely the most consequential for thbusiness. If success is achieved here, it increases the likelihood that treating the bioavailability of IGF-1 may hold the key to treatment of several conditions.
As a rare condition, recruitment will take a while, although Neuren will have maintained involvement with the relevant patient advocacy groups. A clear motivation for patients to join the trial, is the possibility of continuing on after the trial period is complete.
Over the coming months expect newsflow like: 1st patient in, 50% patients in, 100% patients in, trial complete, preliminary readout.
Disc: Held in RL and SM
Jon Pilcher has been teasing investors for some time that $NEU are investigating other conditions that might be treated with NNZ-2591. Today, he's revealed the next target is the SYNGAP1-related disorder (SRD), another rare and intreated neurological condition.
SRD is caused by a genetic variation and patients affected suffer "intellectual disability, low muscle tone, global development delay, epilepsy, sensory processing disorder, gross and fine motor skill delays, coordination disorder, speech delay, sleep and behavior disorder and autism spectrum disorder."
The prevalence is reportedly 1 in 16,000 individuals, which would offer a potential population in theUS of 21,000. Given that there once again no existing condition sets up the product for a high cost of treatment per patient. (Recall, the Rett prevalent population is 5,000-6,000, and DAYBUE looks like it will make peak sales in the US of c. $500m.)
The nature of the condition and the fact that there is currently no treatment marks it out as another potential candiate for orphan designation and priority review. But I am getting ahead of events now.
NNZ-2591 in SRD alone could potentially be a blockbuster (a term from my heyday in pharma in the '90s for a drug that achieves $1bn revenue). Of course, there is likely 4-5 years of clinical development ahead, with all the risk that entails, so don't give up your day job yet if you have one!!
And of course there is also competition risk, with NNZ-2591 more generally being susceptible to any breakthroughs in gene therapies.
But today is a day not to focus on the risks - that will surely come - but to celebrate the fact that NNZ-2591 is truly starting to turn into a single molecule product pipeline.
I'm looking forward to hearing more from this, and from yesterday's news, at the $NEU HY results in a couple of weeks. (They report on a Jan-Dec calendar.)
Of course, the key incremental value-driver is the prgress of the NNZ-2591 Phase 3 trial for PMS (Phelan-McDermid Syndrome). That should keep us all entertained (one way or the other) through 2026!
A good week for $NEU. Yesterday shored up the base(DAYBUE) of my "two for the price of one" investment thesis. Today, has just added a new slide to the upside (NNZ-2591).
Disc: Held in RL and SM

US Pharma company (licensee for DAYBUE) announced 2Q results including DAYBE Sales. The key results are shown below:

I'll give a more complete assessment of the data from the call later this morning, but the bottom line is, this is an excellent result.
In the words of CEO Catherine Owen, DAYBUE is moving "through stabilisation and back into growth".
$ACAD are holding to guidance for the year, but according to my analysis they should comfortably now hit the midpoint of guidance and may well get to the upper limit of even exceed it. They were pressed on this in Q&A and said any modification of guidance will come at 3Q. (There is a history with this product of disappointment, so I think they are being cautious. Catherine clearly is in the "under promise and over deliver" camp. Which is fine with me.)
More later. Also, we should expect a related ASX announcement from $NEU before the market opens and likely a conference call to get Jon Pilcher's take. I imagine he'll be delighted and upbeat.
As for SP action, $NEU has run quite hard over recent months. I'm not sure if this is because any of the instos have insights into the sales via channel checks. Persoanlly, I've not read any research on this. If that is the case then today's sales results explain the SP action.
From my perspective, today's result shores up the base of my "two for the price of one" investment thesis (i.e. de-risks the downside) but probably not enough to justify a valuation update. I'll reconsider this after the $NEU results later this month,
I remain a happy hold here. More detail and analysis later.
Disc: Held in RL and SM
$NEU are holding their AGM today. I was surprised to see the disclosure marked as price sensitive, and so have done a bit of digging to try and figure out the new disclosures.
1. Confirmation of Phase 3 Trial Design and Timing for NNZ-2591 in Phelan-McDermid Syndrome (PMS)
- New detail: The Phase 3 trial will commence mid-2025, and is described in detail:
- 160 children aged 3–12 years, randomized 1:1 to NNZ-2591 or placebo
- 13 weeks double-blind period, followed by 12 months open label
- Two co-primary endpoints aligned with FDA
- Estimated total cost: US$80–90 million, fully funded from existing cash
- Significance: While earlier announcements referenced planning for Phase 3 and FDA discussions, this is the first disclosure of full trial design, size, timing, and funding details.
- Next milestone will be commencement of the Phase 3 trial. (Pending final review and approval of the protocol by FDA. Expected "mid-2025")
2. Hypoxic-Ischemic Encephalopathy (HIE) – Expanded Program and Regulatory Plans
- New detail: Neuren confirms it has planned a Pre-IND meeting with the FDA in Q4 2025 for NNZ-2591 in HIE.
- Also disclosed: the company is exploring a Phase 2/3 trial and identified HIE as a potential in-hospital channel with a repeating pool of patients.
- Significance: While HIE was first disclosed as a new indication in an April ASX announcement, this is the first time Neuren has confirmed regulatory timing, trial strategy, and commercial positioning.
3. Named Patient Supply Expansion Outside Major Markets (Announced by Acadia on 20-May)
- New detail: Acadia has appointed a distributor to facilitate named patient supply for DAYBUE outside North America, Europe, and Japan, potentially including the Pacific region.
- Significance: This specific geographic detail and execution step had not been previously disclosed in ASX filings.
4. Expanded Potential Indication List
- New detail: Neuren explicitly states it is now advancing Prader-Willi syndrome and/or an undisclosed indication, suggesting further pipeline expansion beyond those currently in trials.
- Significance: This is new information indicating potential pipeline expansion beyond the current four NNZ-2591 indications.
My Key Takeaways
There is nothing earth shattering here, however, all the reported developments are positive, so in aggregate, I guess they warrant a positive SP response.
If pressed, I'd say the potential strength of NNZ-2591 as a multi-indication drug appears to be growing over time. Should it be successful in PMS, then that will be the major catalyst for the company. However, patience is required.
I'll admit to be slightly annoyed at the amount of time the Chairman spent bemoaning the share price. The market is the market, and the timelines in clinical development are long.
Disc: Held in RL (5.7%) and SM
The Q1 update from Acadia was very positive. They had previously flagged that Q1 would be a slowdown relative to Q4, based on seasonal effects, so hitting $84.6m revenue from Daybue was pretty positive and sets them up well to hit their annual guidance of between $385-405m. While the revenue chart below looks like an asymptote has been reached, I am expecting this to start increasing again given the commentary around some of the drivers of revenue.
While I consider the numbers to be solid the positive for me were the updates around persistence which is now running at 50% of new starts and has improved considerably since the trials and the commerical launch. It looks like they will have a fairly stable patient population once they get past the 12month mark, with 65% of patients (around 620) have now been on Daybue for >12 months. This is a positive for NEU's continued royalty payments and the likely potential for them to get the $500m sales milestone payment.

The number of patients on the drug has again started to increase after a bit of a softer patch last year. They have 954 active patients up from 870 ending 2024, so they added around 84 in Q1. They still think around 2/3 of the potential patient population haven't tried the drug yet.
They have expanded there on the ground sales team by around 30%, as highlighted last quarter so this should result in higher patient starts now that they move out from the Centre of Excellence Rhett treatment centres. Using the above numbers (84 new starts per qtr and 50% persistence) I can get to the high end of guidance fairly easily without relying accelerating patient growth. Listening to the Acadia call the team seemed fairly comfortable with where they were at and the potential for the next few periods. I think the new CEO has a good sense of the business and has a more polished and focused feel to it than some of the earlier calls i listened too since the launch.
Good news also on the ROW expansion with first non-commercial patient access sales in Germany happening in April and orphan drug designation and a clinical trial in Japan commencing in Q3. So the initial commercial sales milestone ($35m) for Europe are likely in the next financial year and the $15m Japan payment following 1-2 years after.
Overall I'm pretty happy as this had the potential to be a much lower quarter than they achieved. I am not expecting these numbers to move the market but the continued royalty stream really gives NEU the freedom to undeetake the phase 3 trials NNZ 2591 without the potential of a cash crunch
$NEU have issued an ASX announcement this morning reporting on the 1Q 2025 US sales and broader progress of DAYBUE, as reported this morning by $ACAD at the end of the trading day on Wall Street.
I’ll post here $NEU’s overview and then provide my own analysis, having attending the analysts call this morning.
Their Highlights
• Q1 2025 DAYBUE™ (trofinetide) net sales of US$84.6 million, up 11% from Q1 2024
• Record number of unique patients received shipments, up 4% from Q4 2024
• Neuren’s Q1 2025 royalty income was A$13.5 million, up 17% from Q1 2024
• Acadia retained full year 2025 DAYBUE US net sales guidance of US$380 - 405 million, implying full year 2025 US royalty income for Neuren of A$62 - 67 million
• Marketing approval in Europe anticipated in Q1 2026, first shipment of DAYBUE to Europe was made in April 2025 under a Managed Access Program
• Orphan Drug designation granted in Japan
• Distribution agreements now in place to facilitate named patient supply in other regions including Latin America, Middle East and Asia Pacific
• Neuren cash and short-term investments $341 million at 31 March 2025
• Neuren is presenting at Macquarie Australia Conference on 8 May 2025
Further Details from the $ACAD Investor Call
As I will explain in the next section, understanding q-o-q revenue changes is complex. So $ACAD focused on the report that patients treated with DAYBUE in the quarter rose from 920 in Q4 2024 to 954in Q1 2025, i.e., +3.6%.
Last quarter, $ACAD announced they would expand their sales and marketing force by 30%. The reason is that the current salesforce is focused on the high-prescribing Centres of Excellence, with less coverage in the broader community-based physicians who support some two-thirds of the total market. Today, $ACAD reported that the recruitment of these additional sales reps have been completed, and they expect to see the benefit of sale force expansion in the second half of 2025.
At this stage, $ACAD believe that only one-third of the population diagnosed with Retts have tried DAYBUE.
$ACAD also report that now >65% of the 954 patients taking the drug at end of Q1 have been on it for more than 12 months, and that persistency tails off at greater than 50% after 12 months, and thereafter continues to be stable (although I note that no quantification has at this stage been given for what 18 month persistency is looking like. At the next quarterly report, we might reasonably expect to be given a 2-year persistency statistic!)
On tariff impacts, $ACAD report that they “have several years of inventory” in the US, so they are well protected in the short term.
There was some discussion on their exposure to intervention in the US market by the Trump Administration. DAYBUE is a high cost drug and it would exposed if the Administration implements a “most favoured nation” requirement on drug pricing. In essence, such a move would mean that $ACAD would only be able to receive pricing based on prices achieved in overseas markets. The key risk is that if prices in Canada or, say in 2026 / 27 in the EU, are substantially lower than the very high treatment price in the US, then US agreements would be renegotiated. Of course, this is a systemic risk facing the entire pharmaceutical industry, and we are yet to see how this plays out. (Go, the US pharma lobby!!)
Approval in the EU is still expected in 1Q 2026, and $ACAD will soon pass the key 120-day milestone, which is a point in the EMA approval process at which the initial assessment of data is complete. At this point, the EMA can choose to "stop the clock" on its approval process and issue questions to the pharmco. No "Clock Stop" would be a very positive sign for the approval process. So, we should expect a further update on the EU process in the next few weeks. (Remember, any EU approval of DAYBUE in 1Q 2026 then starts the process of country by country negotiation on pricing and reimbursement. Particularly for expensive drugs like DAYBUE, this process itself can take many months and even years!)
My Analysis
The $NEU summary above gives a good overview of what’s going on. Basically, we are seeing steady progress on new patient adds, which remains the key revenue driver.
I want to focus the remainder of this report on the patterns of revenue growth, as there are some important dynamics at play, which are now becoming clearer, and which management have done a reasonable job of explaining.
Patterns in Revenue Growth
Now, the eagle-eyed among us will note that the 1Q-on-4Q revenue decline that was predicted (based on what happened last year, and which took the market by surprise then) has indeed occurred.
The 1Q 2025 sales of US$84.6m were down 13% on 4Q 2024. Interestingly, last year the decline was also around 13%.To provide an overall picture, I’ve plotted the quarterly revenues as well as the percentage changes Q-o-Q (orange bars) and % to PCP (green bars) in the chart below. Early values of the % growth figures are omitted, as they are not meaningful, coming off a small base.

I’d like to dig into this in some detail, as it was discussed on the $ACAD call this morning.
Three things seem to be happening consistently between 4Q and 1Q, as follows:
- Patients pull forward their refills in 4Q from 1Q – potentially ahead of holidays. Management explained that this “pull forward” effect accounted for US$3.5m of revenue being pulled from Q1 into Q4.
- Consequently, scripts and refills are down in 1Q, and there may also be a seasonal effect with fewer patient presentations to clinicians in the winter months
- Net revenue per script falls in 1Q, and this recovers in later Qs due to seasonal patterns in reimbursement, particularly for patients on hybrid Medicare-Private cover.
The first two points are simple to understand. The third requires some explanation. Here goes.
Pharmaceutical companies often see lower net revenue per patient in the first quarter of the year due to how U.S. insurance plans—including Medicare Part D—are structured. At the beginning of the year, many patients haven’t met their deductibles, which means they face higher out-of-pocket costs. This can delay prescription fills or reduce adherence, especially for expensive drugs. In response, companies may provide increased copay assistance, which reduces the revenue they recognize per patient.
Under the 2025 Medicare Part D redesign, the financial burden on drugmakers has also shifted. Manufacturers must now cover a portion of drug costs earlier in the year under the new benefit structure, even before patients reach coverage. This increases rebate obligations in Q1, further lowering net revenue per prescription.
As the year progresses, more patients move past their deductibles and into more stable coverage phases, leading to steadier prescription volumes and fewer financial concessions from manufacturers. As a result, pharmaceutical firms typically see higher net revenue per patient in the later quarters of the year.
So, this 1Q reduction in revenue is a regular feature of both prescribing/refill seasonality and reimbursement/copay dynamics, and little conclusion can be drawn from the revenue number. We’ll need to wait for the 2Q number to better assess whether $ACAD remain on track to hit the annual guidance number. This next result will provide an important baseline against which to assess the impact of the expansion of the sales and marketing force which is expected to start contributing in H2.
My Takeaways
Overall, this is a reasonable result from $ACAD on DAYBUE sales.
The 1Q number is difficult to interpret. It was a bit softer than I hoped for, however, if the expanded sales force has the intended impact, I consider it reasonable that $ACAD have left guidance unchanged. Having said that, I don't have a good quantitative understanding of the reimbursement/copay/Part D dynamics - so I am somewhat blind on that, and have to go with what management are saying. That said, under the new CEO Catherine Owen Adams, communications have been clearer and more consistent from one presentation to the next.
The 2Q number will be more telling, as the bounceback after 1Q will set the stage for the second half of the year, and we have last years number to compare with.
The market has therefore responded reasonably in my view, with SP off 3% at the time of writing. There was little news to react to, one way or the other, after all.
We may see some further reaction based on Jon’s delivery at the Macquarie Conference later today. As each quarter advances, $NEU gets closer to its next milestone payments which would come from any EU approval – or rather first commercial sales in any EU territory which could following during 2026. And, of course, the royalty rate for RoW sales are more attractive than the US.
Overall, $NEU remains on track with my “two for the price of one” thesis.
I was considering topping up after today's result, as I still have only a modest 4.7% allocation. However, even though I have some understanding of the 1Q dynamic, I can't tell to what extent it is masking a weaker overall sales growth. So, I'm going to have to wait another Q.
Disc: Held in RL and SM
Neuren CEO John Pilcher is going to be interviewed live in Melbourne by Monsoon Communications next month (May 20th) for those interested. Last year they did the same and posted it to YouTube..
Nice little article in the AFR today, doesn't add much to information, but lifts Neuren's profile a bit more, which might add a little to some positive momentum.
NEU ASX: This $1.3b biotech is still run out of a spare bedroom in Melbourne
https://www.neurenpharma.com/showdownloaddoc.aspx?AnnounceGuid=97de998d-0017-4778-9610-08e690e7a7ef
A vote of confidence for $153,000
Another Angelman competitor. Starting phase 3 1st qrt 2026. So that is Ultagenix and the new Roche drug. Very understandable why Phelan McDermid went first. Might be tough to recruit for Angelman unless Neuren steps on the gas. I would suspect they might tackle HEI or Pitt Hopkins next to mitigate first mover advantage. I also suspect that Neuren’s FDA primary endpoints agreement has just made other companies end point pathways a lot easier.
FDA and Neu have had their meeting and confirmed primary endpoints for a phase 3 trial
Nice to get some positive news. There was some concern that the FDA cuts would cause a delay. Clearly it hasn't.
No surprises re the trial protocol. All seems reasonable to me. It will be interesting to see how many patients they need to enrol. For Daybue it was 187
It is kind of interesting for me, at least, to compare with Opthea, which I got badly burnt by. Opthea had a phase 2b trial involving 366 patients, randomised and blinded, showing strongly positive results. The two phase 3 trials completely crashed and burned, showing the drug basically doesn't work.
NNZ-2591 did a phase 2 trial for Phelan-McDermid syndrome. It had a total of 18 patients, open label. No control group, no randomization, no blinding. There were similar small trials for Pitt-Hopkins and Angelman syndromes. Results were based on questionaires completed by caregivers and clinicians. Huge opportunity for bias and placebo effect. Everyone is desperate for anything to help these children, who currently have no effective treatments at all. Obviously people are primed to look for positive improvements. Best to take these results with a MASSIVE degree of scepticism IMO
So I'm treating NNZ-2591 as nothing more than a lottery ticket at this stage. Fortunately, as others have said, the company is is probably around, or even below fair value based on Daybue alone.
The phase 3 trial is expected to start around "mid-year 2025"
It will be interesting to see if mass firing of 19%of the FDAs workforce, including the top brass, slows the meetings and information flow down. I suspect yes dramatically and I can’t imagine powerful pharmaceutical companies are going to be happy about potential delays.
With an impending PMS Type C meeting looming we will see if this decision leads to delays for Neu.
God this chaos is exhausting! I am tired I am going to have a lie down.
Ahead of the $NEU FY24 Results call in a few minutes, I put in a question to ask CEO Jon to explain why they seemed to move in late 2024 from expecting the progression into a Phase 3 trial for NNZ-2591 could be managed via email with the FDA to requested a Type C meeting, which will be scheduled for April (from memory).
As part of preparing for the call I include here two items:
- Their 20204 highlights
- My analysis of yesterdays $ACAD call, focusing on progress and outlook for DAYBUE
Overall, I am pleased that the overall outlook for DAYBUE has gone from worrisome, as it was in early to mid-2024, to now a more stable outlook, with the prospect of Europe coming into view.
2024 Results -$NEU's Highlights
- Total comprehensive income for shareholders A$166 million, comprising A$142 million profit after tax and A$24 million foreign currency translation gain
- A$222 million cash and short-term investments at 31 December 2024, A$359 million pro-forma cash adjusted to include receipt in Q1 2025 of PRV sale proceeds, sales milestone and Q4 2024 royalty and payment in Q1 2025 of Q4 2024 tax
- A$56 million US royalty income from DAYBUE™ (trofinetide) in 2024 up 110% from A$27 million in 2023, with guidance for growth to between A$62 million and A$67 million in 2025
- A$445 million cumulative income from DAYBUE over 2023 and 2024
- DAYBUE approved in Canada, first sales expected in Q3 2025
- Trofinetide Marketing Authorisation Application for Europe filed, potential for approval in Q1 2026, Acadia planning managed access programs from Q2 2025
- Acadia commencing small clinical study by Q3 2025 to support marketing application for Japan
- NNZ-2591 potential to address core symptoms of diverse neurodevelopmental disorders, independent of the underlying genetics, supported by positive Phase 2 trial results across PhelanMcDermid, Pitt Hopkins and Angelman syndromes, with other indications under evaluation
- Fast Track designation granted by FDA for NNZ-2591 in Pitt Hopkins syndrome and Rare Pediatric Disease designation granted by FDA for all three syndromes
- Type C Meeting with US Food and Drug Administration scheduled for early April 2025 to discuss primary efficacy endpoints for Phase 3 trial in Phelan-McDermid syndrome, with alignment reached on all other key aspects of the program at End of Phase 2 Meeting
- Preparations continuing for planned commencement of Phase 3 trial in mid-year 2025
Summary of yesterday's $ACAD Presentation
Acadia Pharmaceuticals is positioning DAYBUE as a long-term growth driver in the Rett syndrome market, with strong U.S. sales momentum, a stable patient base, and global expansion plans. The company is investing in commercial resources, patient support, and physician education to further penetrate the market, while its regulatory efforts in Europe and Canada aim to establish DAYBUE as a global standard of care for Rett syndrome.
1. Sales and Revenue Performance
- Q4 2024 Sales: $96.7 million (up 11% YoY, 6% sequentially)
- Full-Year 2024 Sales: $348.4 million (up from $177.2 million in 2023, nearly doubling in its first full year on the market)
- Revenue Growth Drivers:
- Increased bottle use per patient as stable patients titrate toward the recommended dose.
- Higher persistency rates, with 62% of patients on therapy for over 12 months.
- Improvement in patient discontinuation rates (15% QoQ decline).
- 2025 Sales Guidance: Expected U.S. sales between $380 million and $405 million, with 9-16% volume growth.
2. Commercial Progress and Market Penetration
- Patient Base Stability:
- 920 unique patients received paid shipments in Q4, stable from Q3.
- About 30% of Rett patients in the U.S. have tried DAYBUE.
- Persistency at 12 months remains around 50%.
- Prescriber Growth:
- 830 HCPs have prescribed DAYBUE (up from ~800 in Q3 2024).
- 35% of patients treated at Rett Centers of Excellence (COEs), while 65% receive treatment in other settings (community and high-volume institutions).
- Penetration: 55% at COEs vs. 25% in community settings, indicating room for growth.
- Sales Force Expansion:
- Acadia is increasing its field force by 30% in 2025 to enhance engagement with physicians outside of COEs and improve patient and caregiver support.
3. Patient Experience and Retention Strategies
- Treatment Uptake and Persistence:
- Over 60% of patients have been on DAYBUE for 12+ months.
- Titration trends indicate improved dosing adherence.
- Discontinuation Rates:
- Improved by 15% QoQ due to proactive support and education initiatives.
- Support Programs:
- Acadia Connect: Dedicated team providing individualized care and treatment navigation.
- New Initiatives: Direct-to-consumer campaigns, peer-to-peer engagement (patients, caregivers, HCPs).
4. Growth Strategies and Expansion Plans
- U.S. Market Growth Initiatives:
- Increase penetration in non-COE settings.
- Expand physician engagement and awareness efforts.
- Drive growth through omnichannel campaigns, real-world data insights, and peer-to-peer engagement.
- International Expansion:
- Europe: Marketing application submitted to the European Medicines Agency (EMA); approval expected in Q1 2026.
- Canada: First DAYBUE sales anticipated in Q3 2025.
- Other Markets: Evaluating broader global expansion through managed access programs.
5. Forward Outlook
- Short-Term Outlook:
- Q1 2025 Expectations: Sequential revenue decline due to Q4 pull-forward dynamics, seasonal factors, and pricing adjustments.
- Revenue expected to increase in Q2 2025 and beyond as commercial efforts gain traction.
- Long-Term Growth Drivers:
- Increased patient penetration in non-COE settings.
- Global market expansion beyond the U.S.
- Improved dose adherence and persistence.
- Real-world evidence from the LOTUS study to reinforce efficacy and long-term benefit.
Disc: Held in RL and SM
https://finance.yahoo.com/news/acadia-pharmaceuticals-reports-fourth-quarter-210500883.html
A positive result for Daybue sales from Acadia just released. Q4 sales ”$96.7 million and full year 2024 net product sales of $348.4 million”.
This meets higher end of Acadias guidance for the full year.
Hopefully Acadia has set a more conservative full FY 25 guidance this time: “Daybue net sales guidance of $380 to $405 million”
Canada sales and possibly early European revenues should add to this.
Hopefully Neuren’s share price starts to rise accordingly after being beaten down for so long.
Held IRL and SM
Since last Wednesday NEU has gone from a low of $10.90 to today $13.20 or an increase of 22%. This is no micro-cap NEU having a m/cap of $1.4b.
This is all happened on the back of no public news – or none that half-asleep-Scoonie is aware of.
NEU is not an unknown, with every broker in Australia and most of US all over it & Arcadia and NEU being lauded in the Australian financial press numerous times.
One of the more excitable and retail focused brokers have in the 12 months publicly said in reference to NEU: “It's my favourite stock!” Just like selecting a delicious flavour at Gelatissimo.
Maybe that is the issue – NEU has (or had) too many of these investors.
Adding valuation more as a curiousity - Neuren initiated as a buy @ Ord Minnett with a price target of $29.30.
Graham Witcomb from II had an article out today, perhaps a little premature as it seems to be missing some of the updated information from the presentation the company released to the ASX today. None the less it makes the good point that Daybue royalties to 2030 are expected to generate ballpark $1b so the current price also includes success in the research pipeline and/or higher than anticipated royalties from Daybue.
I recommend subscribing to II, but this is the passage which covers the less talked about or well know aspects:
Acadia shakes
While there's no reason to doubt the cash pipes will keep gushing in the short term, Neuren's heavy reliance on Acadia for manufacturing and distribution is a potential weak spot.
A new chief executive, Catherine Adams, took Acadia's reins in September and that could shift priorities to other drugs in Acadia's stable or lead to strategy changes. Neuren has practically no influence over sales under the current licence, which was expanded to a global exclusive licence in 2023, multiplying Neuren's reliance on Acadia.
A management transition may complicate what already appears to be a slowing sales outlook. Acadia's most recent guidance is for Daybue sales of US$340m-350m in 2024—a fantastic sum given the drug was only recently approved, but we can't help but notice it's at the low end of Acadia's earlier (reduced) guidance of US$340m-370m.
Some 30% of diagnosed patients have now tried Daybue, and those patients are almost certainly the most in need and easiest to access, due to their proximity to specialist clinics or recruitment during the clinical trial process. Reaching the next 30% will be a tougher slog, so if Acadia is already lowering expectations, that tells us the drug isn't performing as well as expected. On the bright side, as sales grow outside of North America, royalty margins expand to 20% or more, so Neuren takes a larger cut of net sales.
Competitive pressures are also something to watch. Advancing gene therapies—two of which are in clinical trials—could in time challenge Daybue's relevance. With a single-product focus, Neuren is vulnerable to anything that could disrupt Daybue's status, including post-market surveillance which sometimes reveals safety issues that smaller trials failed to uncover. This is more common for rare diseases with tiny patient pools.
Neuren has transformed from a speculative micro-cap to one of Australia's most profitable biotechs, anchored by a leading product in a high-potential market. While its short-term dividend prospects are strong, the stock's 10-40% premium to projected Daybue royalty payments over the next decade means its investment appeal depends on the success of next-generation drugs. The company only has a few products in the research pipeline and all are in Phase 1 or 2 trials, where failure is the norm. Neuren's current share price appears justified given Daybue's cash flow potential, but the clock is ticking on how long the gushers remain open.
Disc: I own SM+RL
15-Jan-2025: 1:30pm: NEU down as much as -9.17% today (closed @ $12 yesterday and down to an intraday low of $10.90 so far today) with a couple of hours to go. So much for being close to the bottom of their downtrend.
I'm not seeing negatives in today's announcement - Trofinetide-marketing-application-submitted-in-Europe.PDF - but clearly it's below some market participants' expectations. Perhaps it's the pace of the DAYBUE™ (trofinetide) roll-out globally that has some punters thinking that while the growth is there it might take longer to play out than previously expected.
I guess that's the trick. How do you accurately manage market expectations when the market is often irrational and many people are simply following trends or charts without too much understanding of what the business does and the environment in which the business operates?
Pharma is well outside of my wheelhouse, but I know enough to know that trofinetide has been derisked and is going to be rolled out globally, and it's already selling in the US and Canada, and the FDA in the US is regarded by most people to be one of the hardest nuts to crack for pharmaceuticals. And the global roll-out will take time, i.e. years - it won't happen overnight.
I'm bemused, but otherwise unphased. I was obviously back in too early with NEU (in December) and I guess that tends to happen to me more often with companies that operate within sectors that I have less overall knowledge about and experience in (outside my sphere of competency), however when I bought back into NEU in December, it was in my SMSF only, and I'm back to being comfortable with 5 year plus timeframes there now - after my TPD claim got approved and paid in October so my prior need to make shorter term profits is no longer there. I'm back to being a medium-term value investor - mostly - who also likes to make a buck here and there on shorter term opportunities. All good.
Today's Announcement:


Disclosure: I hold NEU both here and in my SMSF.
This is not a full valuation or a bear case, it’s a base case on the currently commercial part of the business to get a feel for what it is worth. On this basis I now have a full position in NEU, with a simple thesis that the current prices is reasonable for just the royalty income from Daybue (as @mikebrisy concluded with much better analysis) and I expect milestone payments to cover the cost of any further research in NNZ-2591 and assume that research fails to generate any commercial value.
Assumptions:
· Duybue sales in NA of US$400m growing at the cost of capital, generate US$43m (A$64.2m) in Royalties, which I view as conservative.
· ROW sales are worth around the same value, so A$64.2m in Royalty revenue.
· The cost to operate the business and for further NNZ-2591 research is covered by Milestone payments on Duybue (ie they net out) including the US$50 for the current milestone and US$50m for the PPV sale.
· PE of 12 and Cash as at 30 Sep of $210m.
· Base Value per share of A$10 per share

Given how rough this is I have bought in at under $13, seeing the downside as minimal. The upside however is significant and I am yet to put any figures on it but each of the following could be worth at least the current value of the company:
· Daybue commercial success above current expectations.
· Fragile X royalties and milestones from Acadia.
· NNZ-2591: multiple opportunities but current Phase 2 candidates for Phelan-McDermid, Pitt Hopkins and Angelman are worth ascribing value too.
I have a lot more work to do on NEU which will take a long time and I had thought I had missed the opportunity when the price spiked, but thanks to RFK’s appointment and the market I am taking this second chance on the basis that this is a very asymmetrical investment in my view.
Disc: I own RL
Neuren seems to have been caught up in the biotech sell off following the appointment of RFK Jnr as the head of the Department of Health and Human Services in the US.
What are everyone's thoughts on this appointment? Seems like the market is looking quite short term towards news that may not actually eventuate to much.
Drugmaker stocks slide as Trump taps vaccine skeptic RFK Jr for US health job | Reuters
Disc: Held IRL and on Strawman.
JP Morgan: Overweight PT $23
Share buyback surprise; focus on first NNZ-2591 Phase 3 trial to start in 2025
Emerging biotechs do not typically find themselves in a position to announce share buybacks. However, for Neuren, the early arrival of a higher-than-expected sale price of Acadia Pharmaceuticals’ Priority Review Voucher (PRV) let them do just that. Neuren announced a $50m share buyback this week, a surprising move but one which speaks to the company’s comfort with its current spending requirements. We are somewhat cautious on what the exact next cost of the phase 3 clinical trial of NNZ-2591 in Phelan-McDermid patients will be, however, Neuren’s current cash position and lean operating model should allow the company to meet requirements comfortably. At this stage, we remain focused on the nearterm catalysts in the development of NNZ-2591 to a late stage clinical asset and view the risk/reward as favourable. Retain Overweight, PT unchanged at $23
• Buyback driven by PRV sale. At Neuren’s current cash position of ~$210m and incoming steady royalties from Daybue, the company maintains it will have sufficient funds to execute on at least two Phase 3 trials (likely to be NNZ-2591 for Phelan-McDermid syndrome and Pitt Hopkins syndrome). We view the announcement of a share buyback as somewhat unnecessary, but understand the rationale of returning excess cash to shareholders
• Clinical trial preparation on track. We await the news of the defined endpoints for NNZ-2591’s first Phase 3 trial in Phelan-McDermid Syndrome patients, likely to be announced in early 2025, for commencement shortly thereafter. Management have maintained the line it will cost US$50-100m per trial and we expect to get a clearer picture once further details emerge of how large the patient size of the Phase 3 trial will be required to be
• Daybue patient adds now steady. Daybue September quarterly sales of US $91.2m was within our expectations, as full year guidance was unsurprisingly narrowed to the lower end at $340-350m. Sales reassuringly continue to grow, lifting 8% QoQ as discontinuations stabilise. Moving forward the focus will be on driving penetration outside the Rett’s Centres of Excellence (more than 70% of patients treated) in the US and abroad, the submission of the European dossier in 1Q CY25. We make limited changes to our Daybue forecasts
• Retain Overweight, PT unchanged. The overhang on Daybue sales volatility appears mostly removed, since the latest Acadia quarterly numbers suggest patient discontinuations have stabilised. Our focus remains on the progress of Neuren’s pipeline drug, NNZ-2591, as it enters late stage clinical trials which should over time demonstrate its platform potential. We continue to see the risk/reward as favourable for Neuren and retain our Overweight rating, PT unchanged at $23 p/share
DISC: Held in RL & SM
$NEU have announced an on-market share buyback of us to $50m.
This is not a surprise as Jon has indicated that the Board have been considering how much cash they need and what should be done with an excess cash.
$NEU were already sufficiently cashed-up to fund the planned two Phase 3 Trials for NNZ-2591, and with a perpetual incoming stream of royalties from DAYBUE, the windfall of $US50m for the PRV sale by $ACAD, has clearly been judged as excess cash, a one-off, to be returned to shareholders.
For long term holders of $NEU, this is an opportunity to deliver some returns. Good to see management exercising discipline.
From my perspective, the return makes sense. I consider $NEU remains materially under-valued, and they won't need the cash for NNZ-2591.
If the strategy evolves and they develop the company to take (in the success case) NNZ-2591 all the way to market, there's probably enough future cashflow coming from DAYBUE to fund that.
Disc: Held in RL and SM
A 34% pop in SP in just the past week on the back of the good DAYBUE numbers - FWIW here's what one broker had to say
MST Marquee: Buy 12m PT $28
Daybue back: Bumper qtr for NEU
MST upgrades CY24 NPAT 13.4%
▪ Daybue grows Q/Q: active patient numbers show pos linear profile
▪ 3Q delivers NEU A$76m each for voucher sale + milestones (2xUS$50m)
▪ Positive progress in Qtr for NNZ-2591 puts NEU on cusp of Ph3 in PMS
▪ MSTe adjusts FY24e/FY25e EPS +13.4%/-9.4% PT $28ps. Buy rating
At 3QCY24, Acadia Pharmaceuticals (ACAD.US) demonstrated a consecutive qtr growth or ‘linear’ profile of active Daybue patients. Despite ACAD’s updated guidance pointing to the low-end of its previous range, NEUe royalties of $54-56m still landed modestly ahead of MSTe. ACAD’s CY24 YTD Daybue sales exceeded US$250m & triggered a further US$50m milestone payment (as MSTe). ACAD’s sale of the Daybue Priority Review Voucher (PRV) adds another US$50m to 2H. NEUe total CY24 rev at ~$216-218m (incl $11m interest). MSTe CY24 rev is increased +11%; we also update for Phelan-McDermid syndrome (PMS) Ph3 R&D costs from 2H25. Our FY24e EPS gains +13.4% but FY25e EPS falls -9.4%. MST PT A$28ps, with longer term FCF profile unchanged
Acadia (ACAD): 3Q royalties, milestone & Voucher sale
ACAD’s 3QCY24 (All US$) net sales for Daybue grew +36.3% on pcp to $91.2m (+7.8% on consec qtr) with growth in unit sales including patient on treatment at ~923 (was ~920 in 2Q). Based on 3Q result, NEU will receive royalties of ~A$13m (10% for sales ≤$250 and 12% between $250-500m). ACAD narrowed CY24 Daybue sales guidance to $340-350m (was $340-370m) implying 4Q royalty to NEU at ~$17m. For 9mths CYTD sales were $251m, above the >$250m for CY required to trigger a further $50m milestone. Separately ACAD sold its rare paediatric PRV for US$150m with NEU to receive ~US$50m
Cashed up: Bal sheet & cash-tax asset from NZ credit
At end 3QCY24, NEU cash and short-term investments totalled A$210.2m, with interest income covering corporate expenses. NEU expects to recognise a deferred tax asset of approximately A$17m in CY24 from New Zealand tax losses, partially offsetting its ‘cash-tax’ given A$12.5m was applied at CY23
Updates: Canada apvl & NNZ-2591 PMS progress
Daybue was approved in Canada in Oct to treat Rett syndrome in patients >2yrs. NEUe TAM for Rett in Canada at 600-900 patients & sales will add NEU royalty payments. On NNZ-2591 NEU says its FDA meeting on key aspects of its Ph3 trial for PMS was positive. NEU plan a 13wk randomised, double-blind, placebo controlled trial in 3-12yrs olds. Ph3 likely starts 2HCY25, but timing and costs remain subject to final efficacy & endpoints
DISC: Held in RL & SM
$NEU paused, presumably while they prepare their release on the DAYBUE sales number. No biggie.
They will however likely now give very tight guidance on FY24 renveue, which will be uplifted due to proceeds from the PRV sales, which $ACAD expects to close in Q4.
So for those in the market who don't understand the details of what's going on, FY24 is going to look strong. It will be interesting to see what this does!
Still writing up my more detailed notes from $ACAD and doing some analysis on DAYBUE.
$ACAD have just announced their Q3 results.
DAYBUE sales were $91.2m, up 36% to pcp and +8% q-o-q, so clearly new scripts are outpacing discontinuations, which is good news.
Guidance is lowered from $340 - $370 to $340 - $350m.
This is pretty much exactly where I expected. Going on the call now.
Disc: Held in RL and SM
Daybue has just received approval for use in Canada. This will add to the US sales numbers.
Lets see if this can halt the continued slide of Neu share price - despite good news.
The recording of today's meeting with Jon Pilcher from Neuren is now up.
The big takeaways:
- NNZ-2591 has the potential to be much more impactful than Daybue -- both in terms of addressable market and the nature of partnership agreements (which should be much more favourable than the initial agreement with Acadia)
- Jon was extremely optimistic about its prospects, not just because of very encouraging Phase 2 results, but because of their experience bringing trofinetide to market
- Expect about 2 years or so before this is contributing to revenues
- In the meantime, they have a fortress balance sheet. It seems more than enough to support them, especially given their Daybue business is profitable and fast growing.
- The partnership agreement outside of North America was significantly better than the initial terms with Acadia, and the market in Europe is essentially the same size.
- The business is in the best shape its ever been, and yet the share price is down 50% or so from its high. I suspect because the market got a little carried away last year, but also because of some doubt cast by a short report targeting Acadia, and a downward revision in Acadia sales -- but Jon said that had little to do with their partnership
Neuren operates in a high-risk space, but it seems like things have been substantially de-risked in recent years. A reliable cash-cow in Daybue with a long runway of growth, a rock-solid balance sheet and another candidate coming to market (hopefully) in the coming years with even bigger potential.
I haven't had a crack at a valuation, but things certainly seem more compelling now given the recent decline in price.
Summary
Following $ACAD 2Q Results in Aug and recent conference presentations in September also by $ACAD, I have firmed up on my recent valuation ($24, $12-$46), and am getting more comfortable with the future US sales trajectory for DAYBUE.
As a result, I've added back most of the shares I sold in May at yesterday's price, which I consider a bargain.
I consider that today the market is offering $NEU on a "two for the price of one" basis, ... or thereabouts.
Context
With all the focus on ASX reporting over recent weeks, I’m only now catching up with some of the wider research opportunities in my portfolio. One area I am giving some focus is $NEU. I’ll not repeat information here from earlier straws and posts, but in summary, this business has two important things going on:
1) DAYBUE being sold under licence by $ACAD in the US, with work underway to gain approval in Canada (likely end-24/early-25) and EU & Japan (approvals likely only in 2026+)
2) NNZ-2591 has completed Phase 2 studies in three neurological conditions, with an end of Phase 2 meeting with the FDA for Phelan-McDermid syndrome due this month. (This is likely the first indication to advance to Phase 3, given the US market potential of 17,000-32,000 patients. While there's still clincal development risk, it's potentially much bigger than DAYBUE)
We’ve covered NNZ-2591 in other recent straws/posts. My focus here is to consider my view on DAYBUE in the light of recent communications from $ACAD, including over the Summer Conference season in the US.
I’ve given a rough valuation of $NEU as $24.00. Being $16.00 due to DAYBUE and $16.00 due to NNZ-2591, risked at 50%. In my normal way of presenting valuations, I have this down as $24 ($12-$46).
This compares with analyst views of $26.56 ($22.90 - $29.90, n=6).
This picture below shows the SP progression relative to the consensus view, which suffered a modest downgrade in August, due to $ACAD downgrading guidance for 2024 DAYBUE sales.
So, we all know that momentum players in the market hate downgrade cycles, and so a very large gap has opened up between valuations of the business and the SP.
Hence the title of this straw: I view that with $NEU you are essentially getting a DAYBUE business (which I see as worth $16) and a success case for NNZ-2591 (which I see as worth $16 unrisked) for $13.59 at the time of writing. The analysts agree – two for the price of one!
The reason I sold down 1/3rd of my RL holding in $NEU on 15-May was that I was concerned about the 1Q report from $ACAD. According to my model, $ACAD were never going to hit 2024 guidance for DAYBUE, and I didn’t buy the offered story about a harsh winter causing clinics to shut down. But as importantly, I became suspicious of management – having prematurely offered annual guidance, and then spinning a 1Q story and acting as though things were on track for the year.
Roll forward to the 2Q report in August. While sales began to recover, guidance was indeed finally lowered, and that pushed many ASX shareholders into a funk with shares sliding from c. $18 to $13-$14.
To put this in context, you can buy $NEU today for less that you could at the launch of DAYBUE, which pre-dated the stream of positive newsflow on NNZ-2591.
So, What Can We Say About DAYBUE?
Having gone back over the $ACAD 2Q Results call (8-Aug), the Morgan Stanley Healthcare Conference (4-Sept) and the Baird Conference (11-Sept), I have firmed up my view on what is happening with DAYBUE. (BTW, recordings of both conferences are accessible on the $ACAD website.)
It is now clear to me that what happened following the launch of DAYBUE: $ACAD were completely surprised by the demand in the first 5-6 months. In retrospect, this should not have been surprising. Rett Syndrome is a very challenging condition, and there is a strong global Rett community. With no pre-existing treatments on the market, there is a well-established specialist support network via Rett Centres of Excellence that support about 25% of US Rett patients. Highly motivated patients, including the parents of sufferers who carry the burden of care, quickly accessed their HCPs requesting the product as soon as it was launched.
This drove strong initial sales in Q3 and Q4 2023, and it led $ACAD to prematurely issue what now turns out to be overly-optimistic guidance (… which was clear to me as early as May).
But as we know, DAYBUE is not well tolerated by many patients. So Q1 and Q2 2024 saw two things happen. 1) The initial “surge” from "c. 25%" of the potential market subsided and 2) discontinuations from this initial “bolus” swamped new patient adds. Aaaaah! DAYBUE has stopped growing! Sell!
This complex dynamic made the interpretation of data from Q2 very challenging. But as a new normal in net patient adds began to establish itself, $ACAD were able to reset 2024 guidance to a more realistic level.
Why Am I So Interested in What $ACAD have been saying in September?
With the “panic spin” of the 2023/24 winter behind us, I have been forensically examining $ACAD's statements throughout September, to test them against what was said in August. I can bring myself to overlook what I consider as “Winter Spin” because, indeed, the dynamic – while easy to model after the fact – must have been very disorientating at the time. And who knows, maybe winter clinic closures and delays in reimbursement renewals for refills created a genuine “winter fog”. Management word salad didn't help, but perhaps I was too quick to judge.
What matters is the future and whether $ACAD are properly characterising the current performance, and the path ahead.
So Where Are we Now?
Throughout the last three public disclosures, a consistent picture is emerging. Here are the key points.
Persistency in the real world remains about 10% ahead of clinical trials. This has been a consistent story for 2024. The best estimate of long-term persistency remains around 50%
Patient adds are net positive, and they are coming into line with the patterns $ACAD say they more typically expect to see in rare disease treatments
A consistent picture is emerging for each of the HCP segments:
1. COEs (25% patients) – 50% penetrated, 1/3rd of prescriptions to date. Major area of focus, as these continue to attract new patients. (My thesis is that the availbility of a treatment might increase diagnoses, expanding the market from 5,000 current to the 6,000-9,000 estimated prevalence. CoEs will likely attract an outsized share of new diagnoses.)
2. High volume non-COEs (60% patients) – “good penetration” and the current area of focus
3. Low volume HCPs (25%) – many treating only 1 or 2 Rett patients – large number of HCPs.
Overall, c. 30% of the total diagnosed Rett populated has started treatment on DAYBUE and, by my calculations, Q2 revenue represents an annual revenue run-rate of c. US$340m.
Against the updated (downgraded) guidance for 2024 Revenue ($340-$370m), $ACAD have said at the September conferences that they are tracking to “just below the midpoint of guidance” which I interpret as being c.$350m.
They’ve also said that they see “more upside than downside” from here, which says to me that they are seeing the guidance offered in early August as still appropriate, and we are now getting to the closing phase of the year.
My Analysis
The message consistency across three presentations spanning 5 weeks, indicates to me that $ACAD have got to grips with DAYBUE sales performance. Things seem stable.
I see the journey of the 16 months since launch in three clear phases:
Phase 1: The Pent-up Demand “Bolus Surprise" - April-October 2023
Phase 2: “Winter of Discontent”: Early Patient Discontinuations swamp new scripts - Nov 2023 – March 2024
Phase 3: “Stabilisation” – Normal Market Penetration and Steady Growth - April 2024 onwards
Looking further ahead, while, based on my model, $NEU are unlikely to get their 2025 $500m US milestone, this is likely to come in during 1H 2026. Canada might give 2025 royalties a bit of a push, as it experiences its own “mini-bolus”, and then hopefully Japan and EU will add new impetus to revenue with milestones and royalties in FY26 and FY27.
So, overall, I’m increasingly comfortable about the downside floor to the SP being in the $low-teens. Which is where we are today!
So, my original investment thesis is intact. Today, I can buy $NEU for less than the fair value of DAYBUE, with the free option of the upside of NNZ-2591.
Investment Decision
My valuation remains as published previously. However, yesterday in RL I have added some $NEU to my portfolio, getting back to 94% of my original position. I’ll potentially add some more, but at just over 5% in RL, I’d also be happy to settle here.
Near term upcoming news flow is:
- Readout from September FDA meeting on NNZ-2591
- $ACAD Q3 results in mid-October
Disc: Held in RL and SM
Always nice to see management or board members investing in their own business.
Announcement out Friday Director Joseph Basile just paid $100k of his own money to buy beaten down shares.2A1541407_NEU.pdf
@mikebrisy @Nnyck777 FWIW this out from JP Morgan this morning - it basically highlights things you've already surfaced in your recent posts
Angelman results establish NNZ-2591 platform potential
Neuren has now delivered its third set of promising efficacy data for NNZ-2591, extending the range of rare genetic neurodevelopmental diseases potentially treatable with its key pipeline drug. The data presented for Angelman syndrome (AS) suggests it has potential to progress to the next clinical trial stage. However, current competitive dynamics in AS means it will be prioritised behind other indications. Of immediate focus will be NNZ-2591’s next steps in Phelan-McDermid, where Neuren will be engaging with the FDA next month in a post- phase 2 meeting, which we are hopeful will yield the progression of NNZ-2591 to a phase 3 trial. We continue to see upside in the NNZ-2591 platform and have lifted our DCF-based price target to $23, Overweight retained
NNZ-2591 “multi-indication platform” viability strengthened with promising Angelman results. NNZ-2591 delivered CIC and CGI-I mean scores which were clinically meaningful in its phase 2 trial of AS. This is the third neurodevelopmental disease to have shown early efficacy signs with NNZ-2591, following Phelan-McDermid in Dec-2023 and Pitt Hopkins syndrome in May-2024. The promising data set suggests the potential for other genetic neurodevelopmental diseases could be treated with this platform treatment
Two other competitors ahead in Angelman. As previously covered NNZ-2591 faces competition in AS with the potential of two competitors entering phase 3 clinical trials within the next 12 months (Ultragenyx & Ionis Therapeutics). It is too early to assess which treatment is leading and we caution comparing results across earlier trials. However, should NNZ-2591 progress, it has the administrative advantage of being an oral liquid dose vs the competitors intrathecal (in the spine) dosage
Competitive market dynamics mean Phelan-McDermid and Pitt Hopkins will be prioritised. Neuren have indicated it plans to focus on Phelan-McDermid and Pitt Hopkins after the good phase 2 trial results recognising the lack of competing therapies currently in trials. Neuren’s existing cash reserves will allow it to support two phase 3 trials itself (each to cost US$50-100m)
What’s ahead? While the newsflow has slowed with no further phase 2 trial read-outs for NNZ-2591, next month’s post-phase 2 meeting with the FDA for NNZ-2591 in Phelan-McDermid will be important. An ideal outcome would be the announcement of a phase 3 clinical trial noting Neuren will only comment once the meeting minutes are released. On Daybue, although the weaker guidance was a disappointment, we look to market expansion as sources of upside in the year ahead starting with the Canadian market later this year and Europe in early CY25
Retain Overweight. We lift our probability weighted valuation for Angelman syndrome which brings our Jun-25 price target to $23
DISC: Held in RL & SM
FWIW from JPM this morning (before the Angelman results were released)
Softer Daybue sales, attention likely to shift to drug pipeline
Daybue royalties in the June quarter missed our estimates due to slower new patient starts than expected. We have cut our 2024 royalty estimate by 14%, but we do not expect the milestones payment to change, leading to a 7% reduction in 2024 revenues. The lower revenues were offset by lower costs and higher interest income, leading to minimal changes to our EPS estimates. While the slower Daybue uptake is disappointing, the reduced cashflow should have no bearing on Neuren’s R&D pipeline plans. We remain confident Neuren will progress its NNZ- 2591 pipeline, noting the impending phase 2 read-out for Angelman syndrome and End of Phase 2 meeting with the FDA for Phelan-McDermid syndrome next month. Our PT reduces to $22 (from $26.70) on lower Daybue royalties; retain Overweight
DISC: Held in RL & SM
I've now gone back over the recording of the $ACAD announcement with a fine tooth comb.
Contrary to my initial impression, listening a little (i.e. A LOT) more carefully, I felt there was less evasion and more just some incompleteness and relatively minor inconsistencies. More a "misty day" than a heavy fog or smoke screen. So, I do need to row back a little on some of my less generous remarks about the management team.
I still believe there wasn't a straight answer on the active patients as at 30-June. However, I can understand from my own experience why a management team would agree not to put out such data for 30-June and 1-August, which covers the Summer month of July, to mitigate the risk that some idiot analyst would multiply the difference x 12 to get a misleading annual growth number. I.e., discontinuations don't slow down, but new scripts do over the summer month. Fair dues, I have also been guilty of managing similar messages when on their side of the table. And we all have our our experience here in Australia of the "Dance of the Seven Veils" with DW at $PNV.
OK, now I've got that off my chest, on with my analysis and key takeaways.
We've had a lot of posts on $NEU from several members with a lot of data and sound bites, so I'll not repeat any of it. Rather, I will lay out some calculations, based on the US alone, and then use that as a basis for further discussion of value and risk.
Methodology
I have decided to use a range of M&A mutliples of forecast peak sales to set out a range of scenarios for the value of DAYBUE in the US to $NEU.
Method is as follows:
- Market size (A): base 5,000. Range 6,000-9,000,... so conservatively I am taking scenarios of 6,000 and 7,500
- Long Run Persistency (B): 50% - there is upside and downside, but I'm keeping that simple
- Peak Market Penetration (C): scenarios of 40%, 45% and 50% (we're already at 30%-33%)
Revenue Per Patient (D) : As a shortcut, I used an RBC Capital Note from June 2024. They set out their calculations using dosing assumptions and cost per patient per annum of $585k and a gross-to-net leading to $536k per patient per annum.
The reason I chose RBC Capital, is that they appear to be a House Broker, who undertook or commission some detailed market research to support the valuation. I'm therefore not accepting their market assumptions, as I think they might be biased, but there dosing and revenue per customer assumptions appear to be OK.
Continuing the Method:
- Calulate Peak US Revenue: (PRev) = (A) x (B) x (C) x (D)
- Calculate $NEU Royalty Payments from tiered US Royalty Schedule (Roy)
- Apply Peak Revenue Multiple: (M)x(Roy)
- Add Sum of Milestone Payments (SMP)
- Convert to $A
- Value of US DAYBUE to $NEU = (M)x(Roy) + (SMP)
- Divide by Shares of Issue
- Result is an estimate of the $/Share of $NEU attributable to the Payments from $ACAD due to US Sales of DAYBUE
Acquisition Revenue MultipleBenchmarks
For the acquision revenue multiples, I have considered a wide range.
I've rejected more spicey multiples of forecast peak revenues in biotech, which can get up to 12x to 20x and more, and this is perhaps an area requiring further consideration.
Having examined some benchmarks, the reasonable range for a pharmaceutical company with a fully commercialised product in the market is an EV/Forecast Peak Revenue multiple ranging from 5 to 10.
In any event, it is simple enough for you to form your own view.
Here are the calculations:

So What? (Part 1)
If I assume that DAYBUE gets to a peak 45% market penetration within the next 2-3 years, so as to attract an acquisition multiple of 7.5 x Revenue, then the revenue stream to $NEU could be valued in the ballpark of $7 - $12.
Now I have to allow for Canada, Japan, EU and RoW - should these eventuate. These have a more attractive royalty structure, however, they are likley not to be as material in aggregate as the US. Let's assume that the better royalty structure is balanced by the small underlying aggregate revenue, so that Peak RoW equals Peak USA.
Assuming Peak ROW occurs 4 years after Peak USA, then by the same method, its worth $5 - $8 /share
This means the value of DAYBUE to $NEU is $12 - $20 - or $16 at midpoint.
But What About NNZ-2591
NNZ-2591 could be worth $0. But it could be worth 3 x DAYBUE, but another 5 years into the future, so let's say it could be worth 2x DAYBUE today.
My Decision
Who knows what the market will do tomorrow. But now, I just don't care.
My investment thesis is that $NEU is worth the value of DAYBUE, giving me a free option to the Upside of NNZ-2591.
If tomorrow, $NEU tanks 20% to below $14, then my thesis is completely intact, because I believe even given the less-than-stellar performance of DAYBUE, $NEU is worth anywhere from $12 - $46.
There is still uncertainty around DAYBUE, but it is rapidly becoming de-risked with now 9-months of Real World persistency data, and the growing evidence of open label clinical extension data spanning 3 years.
This is precisely the kind of risk I still want in my portfolio. I'm so grateful for the Angelman Trading Halt, because it has given me the time to do a proper analysis.
In fact, if the market throws a tantrum tomorow, I will buy back the one-third I offloaded on the back of the 1Q result.
I'm laying this all out in detail, as I value the views of the other StrawPeople who are following this one closely. (I won't hold my breath while you find the obvious errors!!)
Disc: Held in RL and SM
P.S. I have referred to the work of $ACAD House Broker RBC Capital. While they have a bias that is plain for all to see, they are one of the few houses I have seen that has done a detailed market analysis, based on primary research. Their reaction to the $ACAD result was to mark it down from $29 to $26 vs. closing SP of $15.17. The bias is evident in their elevated valuation; however, the fact thay they only marked $ACAD down by $3 or 11% from their elevant elevated valuation is telling - it is in line with my own view based on entirely indpedendent analysis - apart from the $/patient assumption.
Neuron’s value lies in:
1) Both Trefinitide (Daybue) sales in the USA and potentially in RoW
2) NNZ 2591 – This has (currently) four identifiable valuation parts: Phelan McDiarmid (PMS), Pitt Hopkins (PTHS), Angelman’s and Prader Willi conditions. Prader Willi is still in Ph1, so ignore for now.
Friday’s upcoming announcement for Angelman’s Ph2 results are likely of lesser relative valuation importance because:
i) Angelman population size is not as large as the PMS condition (the most promising). It is about 60% the size, though larger than PTHS.
ii) With respect to Angelman’s drug development, there are other companies ahead of NEU.
John Pilcher last month stated NEU’s priority will be in progressing Phelan McDiarmid (PMS) and Pitt Hopkins (PTHS). (JP emphasised just how important it was to get FDA approval and get to market first).
PMS is particularly favoured for development because:
- it has a sizable market (1,700 known patients though potential 17,000 to 32,000),
- they reported excellent Ph2 results
- with PMS (and PTHS) they are ahead of the competition.
How the market reacts to the Angelman’s Ph 2 results when muddied with Acadia’s recent sales results is anyone’s guess. A good Ph 2 results will be of course a positive, but it is not the main game.
This quarter NEU will meet with the FDA for feedback on NNZ 2591 for PMS. If positive, then the Ph 3 trial will likely take a little less than two years. So it will be a slow grind – with lots of periods for investors to become alternatively bored and nervous.
However NEU has a number of further attributes in its favour which the market seems to be losing sight of:
- NEU have $240m in the bank and no debt. NEU have enough funds undertake the PMS and PTHS Phase 3 trials themselves. As a scale example of what might be needed should they choose to do so, Arcadia have around 50 staff working on the whole Daybue enterprise.
- they have ongoing royalty revenue from Daybue in the US. (It may be less than many investors expected - however at around say $50m/yr is way more than most smaller drug companies have).
- The RoW Daybue rights have been competitively bid and won by Acadia, on better terms for NEU. It is possible with a few things going right, NEU might see in two to three years similar royalty revenue from RoW sales.
- John Pilcher and NEU has done it all before with Rhetts syndrome. Admittedly Arcadia carried the later stages - but NEU understands how to engage with the FDA, the Community Groups and know the US distribution and reimbursement landscape. These are not first-timers who are whispering sweet nothings in your ear. They have done it before.
- The PMS results at Ph2 were better than Trefinitide /Rhetts at the same development juncture.
- The CEO John Pilcher is smart, dogged, honest and knows his way around.
- NNZ2591 does not suffer from the major clinical handicap of Daybue - NNZ2591 has no reported diarrhea side effects.
Likely NEU is either going to be bought out or in around two years time, with a little luck, you might find yourself up two or three times on your money. But who really knows.
$NEU is into a trading halt as it prepares a presentation on the top line results of the Angleman Phase 2 Study for NNZ-2591.
This is tricky timing, as buying and selling was setting up a 10% fall in SP based on the DAYBUE result.
Shareholders looking to exit may be bothered that they weren't given the chance to get out early. By the time trading resumes (presumably Friday morning) analysts will have given reports on DAYBUE sales, which as I wrote at length is complex and likely disappointing.
Personally, I'm pleased, as it gives me some time to properly digest the DAYBUE result and then consider what weight to give to NNZ-2591 in light of the Angleman result, taken together with the previous results.
This was tricky from an investor relations perspective - not allowing the market to react to reported results. Some investors may take cues from $ACAD SP response tomorrow. However, $ACAD is a multi-product company, where the other product was upgraded. However, there may be a range of analyst commentary to consider before taking decisions.
How might this play out? A strong Angleman result would unlikely on its own offset a 10-20% decline based on DAYBUE. However, as @Nnyck777 has stated before, a positive read across all indications (i.e., a less stringent regulatory pathway due to the larger dataset) could potentially more than offset DAYBUE softness.That said, I'm not sure how good the market will be at assessing the read across - i.e., what risk to attach to it.
Ho Hum.
FWIW JP Morgan (Overweight with 12m PT $26.70 - so take this with the requisite grains of salt) have released an updated note with the following summary highlights ...
Thoughts ahead of the Angelman phase 2 results in 3Q24
Expectations are high ahead of the results from the phase 2 trial of NNZ-2591 for Angelman syndrome (AS) due early this quarter. A positive result will support the belief that NNZ-2591 is a “multi-indication platform”. In this note, we focus on what to look for when the phase 2 results are released, along with an assessment of the competitive landscape. AS is an attractive opportunity as there are currently no approved therapies but the competitive challenge is greater than for Phelan-McDermid and Pitt Hopkins syndrome where NNZ-2591 has the potential to be the first therapy to market. Beyond the phase 2 results for AS, Neuren remains well positioned with catalysts ahead including the quarterly result and an FDA meeting. We reiterate our Overweight rating
Focus on CGI-I and CIC endpoints for Angelman. These two efficacy endpoints will give a sense to how much NNZ-2591 reduces disease severity in AS patients. Neuren hopes to deliver scores below 4 and ideally less than 3. While the positive results from Neurens previous trials of NNZ-2591 in Phelan-McDermid and Pitt Hopkins syndrome were encouraging, we caution the read across to the Angelman trial is limited given its different etiology
Neuren’s Angelman trial has been completed with results due this quarter. The last patient enrolled for the AS trial was about two weeks after the last patient enrolled for the Pitt Hopkins trial, which reported results last May. However, we expect the collection of results and analysis of data to take longer with AS because the clinical trial sites were in Australia which have less experience compared to US sites
Several competitor trials for Angelman. AS presents a sizeable opportunity with no current therapies. We are aware there are other therapies in trial which are all at a similar stage to NNZ-2591, including Roche’s GABA-modulator alogabat and antisense-oligonucleotide candidates Ultragenyx’s GTX-102 and Ionis Pharmaceuticals’ ION582. These trials are also early stage but Ultragenyx indicated it plans to initiate a phase 3 trial by the end of 2024
FDA meeting for NNZ-2591 in Phelan-McDermid plus new indications. Also this quarter will be Neuren’s post phase 2 Phelan-McDermid meeting with the FDA. We expect the company to make an announcement once the minutes from this meeting are published. We are hopeful this will support the move to a company funded phase 3 trial starting in early CY25. We also expect the company to announce its plans for NNZ-2591 in the treatment of other rare neurodevelopmental diseases
Retain Overweight. Daybue sales in the June quarter (to be reported by Acadia Pharmaceuticals in early August) are difficult to predict after the volatility in the previous quarters (strong Dec but weak March) with this uncertainty weighing on share price. We are comfortable sales will lift over December but would see any disappointment as an opportunity ahead of the NNZ-2591 results
DISC: Held in RL & SM
Note: Don’t hold sold out a few months back. I am interested in getting back in.
Are the risks that Phase 3 results are not as good as people are expecting? Digging into the Phase 2 results showed (see below).
I don’t know it’s not exactly what I would say shooting the lights out. Just trying to get a sense if it’s worth getting back in.
I just had a feeling the report (Phase 2 trial shows significant improvements in Pitt Hopkins) released 27/5 wasn’t as up beat as previous.


$NEU announced the initial read-out of it Phase 2 Clinical Trial of NNZ-2591 for Pitt Hopkins syndrome.
Their Highlights:
- Statistically significant improvement from baseline assessed by both clinicians and caregivers in all four efficacy measures specifically designed for Pitt Hopkins syndrome (Wilcoxon signed rank test p<0.05)
- Clinician and caregiver global efficacy measures showed a level of improvement considered clinically meaningful:
- Clinical Global Impression of Improvement (CGI-I) - mean score of 2.6, with 9 out of 11 children showing improvement assessed by clinicians
- Caregiver Overall Impression of Change (CIC) – mean score of 3.0, with 8 out of 11 children showing improvement assessed by caregivers
- Improvements were seen in clinically important aspects of Pitt Hopkins syndrome, including communication, social interaction, cognition and motor abilities
- NNZ-2591 was safe and well tolerated, with no serious or severe adverse events and no meaningful trends in laboratory values or other safety parameters during treatment
- Second positive Phase 2 trial result further strengthens confidence in NNZ-2591's potential relevance for multiple neurodevelopmental disorders
The webinar is at 11:00 today, but in advance of that, these look like very strong results to me – even given the small sample size.
My Assessment
The CGI-I mean score of 2.6 compares with the score of 2.4 in the PM trial, so only slightly less strong, and the difference between the two is not statistically significant.
Equally, the CIC mean score of 3.0 compares with a score of 2,7 in the PM trial. Again, slightly less strong, but again the difference is likely statistically insignificant.
From a physician's perspective 9/11 children showed improvement and from a caregiver prespective, it was 8/11. That's very positive.
See graphical analysis from the presentation below:


So that’s two-from-two at this stage.
We have to recognise that it is still early days for NNZ-2591, with years until we have a commercial drug in the market. But this is very good news, from my reading of it.
NNZ-2591 is currently undergoing clinical development for four rare neurological conditions at Phase 2 clinical trial level. All programs have been granted Orpha Drug designation by the FDA. The results of the trial for Phelan-McDermid were reported in December, showing significant improvements in that condition. The next cab off the ranks is Angelman syndrome, for which the company announced completion of enrolment of subjects in December 2023. This trial is expected to report in Q3 2024. The final of the four, Prader Willi, opened its first site in June 2023, but I don’t recall having heard further information on progress.
Implication for Valuation
Clearly positive.
With potentially 10,000 patients in the US, NNZ-2591 for PW alone could be another DAYBUE, albeit discounted off into the future by 3 years, and perhaps discounted by 50% as the CoS from here. Still, if you value DAYBUE around $20, and you assigned no value to PW, then today you might add anywhere from $5-$7 of SP value. Let's say NNZ-2591 has already recognised half this value for PW, then you'd see the SP rationally increased by $2.50 - $3.50 off today's news. We'll see.
In any event, the market will clearly respond positively to this news.
I recently reduced my exposure to $NEU by one-third, given that I have marked down the valuation of trofinetide based on the latest DAYBUE sales. Clearly, with four potential conditions to treat, the ultimate value of NNZ-2591 may dwarf trofinetide, and today’s result makes me feel more bullish about the prospects for $NEU.
I need to mull this one over. Such it the rollercoaster of drug development, that there is time for a considered response after the heat of the day has passed. $NEU remains by 7th largest RL holding, although after today I imagine it will pop up to 6th or maybe even 5th.
Disc: Held in RL and SM
$NEU into a trading halt today, as some data has emerged from the Pitt Hopkins P2 clinical trial (I think this is the one that was expected in Q2 2024 for NNZ-2591).
Depending on what is actually being announced, I imagine there will be a release and potentially a presentation on it tomorrow. (A presentation if its a trial initial read-out.)
Disc: Held in RL and SM
As the $NEU SP enjoys another SP pop on the back of no new information at a conference this morning, I thought I'd air something that has been bothering me since I analysed the results of DAYBUE in detail over the weekend.
HEALTH WARNING: the charts I have posted below are the just the results of modelling. Even historical data are not disclosed results and there are discrepancies with disclosed facts. So the analysis should not be understood to be my forecast.
With the disclaimer out of the way, I realised I cou;=ldn't fully reconcile the DAYBUE Q1 sales results with some of the statements made on previous calls. Understandably, with the product in the market for only one year, $ACAD are being careful.
Top Level Question: Can DAYBUE sale reach 2024 Guidance (The market seems to think it can with only modest downgrades to TP's for $ACAD and $NEU)
Facts: What do we know?
Sales Revenue ($US m):
Q223 $23.2
Q323 $66.9
Q423 $87,1
Q124 $75.9
Patients Taking the Drug
Q223: not disclosed; 400 prescribers had written scripts, with 7/10 so far converted to paid prescriptions
Q323: about 800 patients taking the drug
Q423: almost 900 patient taking the drug (ok, I assume 890)
End-Feb: 860 patients taking the drug
End-Mar: 862 taking the drug of 1300 who have initiated.
Other Key Facts
Over the winter holidays there were payer delays with getting refills.
January: significantly reduced Rett Clinic days in over 50% of COEs
January-February: discontinuations peaked (due to massive uptake in Q4) exceeding new starters, hence net patient declines.
For the 6 weeks up to 3-May, net patient adds positive again for each week (which means net declines continued to late March!)
We know the Persistency of the drug over time. Below is my modelled curve which interpolates gaps in published data.For the purposes of this analysis we can treat this as a fact (even though there is long term uncertainty).

Modelling Method
With all the above information, I have built a simple model as follows.
Patients (month m) = Patient (month m-1) x Persistency Function + New patients (m)
Guess and iterate patients in months 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11 to fit reported numbers
Ramp down new patients in month Dec, Jan, Feb and March to hit reported numbers
Ramp back new patients from May 24 and hold new adds constant to match company statement that we are entering a more linear phase (this was the report at Q4 results and essentially repeated most recently).
So what will the rate of new patient adds be? That's the billion dollar question.
In the scenario below, I've modelled 88 per month. That would take them from 1300 patients having started the drug by end April 2024 (26% of the US diagnosed population) to 2000 (or 40%) by end 2024.
Note: you can't do the maths without the model because you need to apply the perisistency function to each cohort.
Here's what it looks like.


Revenue Calculation
This is the hairy bit. I do a prop rata calculation on the quarterly revenue based on the average modelled number of patients taking the drug in the quarter and BINGO. (I could have been more sophisticared and taken the unit price x n x 75-80% average rate, but I am nore sure that this leads to a better answer. Add to the "to do list".)
For the scenario shown above, my modelled 2024 DAYBUE revenue is: $US346m (versus guidance of $370m to $420m)
So, if I believe the model, the question is what level of monthly new patient adds do I need to hit the bottom of guidance.
Answer: 116
This gives end 2024 patients of 1428 with 2225 having initiated, which is 44.5% of the diagnosed population.
If they can sustain that rate of patient adds, they'll get to 2313 patients by end 2025, with 3613 patients having initiated or 72.3% of the diagnosed population.
I won't model higher rates because I don't think its credible. Here's why. Reaching the entire population will be a challenge. My scenario above assumes linear growth is sustained, which is very unlikely into 2025 as you start to penetrate the second half of the population. (New adds will follow some kind of exponential decay function).
One reason uptake will fall off, is that a significant proportion of the population will likley elect not to try the drug. Side effects and limited efficacy will put some off. Furthermore, there will be accessibility issues.
Implications
First, maybe my modelling is wrong. But do far I haven't found the bug.
Second, maybe my revenue calculation introduces signfiicant error. After all, the model predicts that Q124 revenue should have been $88m, whereas is was $76m, but maybe they really got hit bad by reimbursement delays over the winder holidays?
But if the model is right, why didn't $ACAD update guidance? Who knows. Maybe they want a clear quarter, so they can update when they report in August. But by then if my model is right it will be very, very clear. So continuous disclosure obligations would push them to announce an earlier update.
$ACAD have better data than me, and would be able to build a much better model. (Mine only took an hour's work!)
The Bear Thesis
DAYBUE sales were blamed on seasonal effects impacting: 1) reimbursement and 2) new patient adds.
The model, which is pinned to reported data, help visualise how significant this must have been. It is interesting to re-read the transcripts with this picture in mind.
$ACAD might just hit bottom end of guidance. Too early to tell. But likely guidance will be revised downwards, once they have a better handle on monthly new patient adds. They only have 6 weeks of stabilisation at the last call, so maybe fair enought.
Implications for $NUE
2024 - the US$50m milestone is safe, so that's great news.
2025 - the next US$50m milestone will just be hit in 2025, if $ACAD hit 88 new patient adds per month and sustain this into 2025. If new patient adds fall in 2025, then it will miss.
Canada will help royalties for 2025.
My rough forecasts for $NEU as follows (consensus in brackets):
88 monthly patient add:
2024 $130m ($152m); 2025 $163m ($172m)
116 monthly patient adds (to be clear, I consider this scenario unlikely, based on the modelling)
2024 - $134m and 2025 - $185m
Implications for the Investment Thesis
The modelling confirms my hunch that the $ACAD results point to more than a seasonal blip.
That said, with Canada, Japan and EU in the pipeline, DAYBUE could still become a $bn+ drug by 2027 / 2028.
However, the weight of my thesis has shifted squarely over to NNZ-2591.
The result on the Phase 2 trials due in Q2 and Q3 this year will guide my portfolio weighting.
In the interim, I am bracing myself for an $ACAD downgrade - which would knock the SP.
On balance, my sense of risk-reward has shifted, and I have taken the opportunity today to trim my position size by one-third.
Investment Strategy
I now have a model to which I can history match the Q2 result. This will add a lot to my insights and projections.
If NNZ-2591 continues positive results in future indications - will add back to my full position as SP allows.
Likely catalyst to add back will be the $ACAD guidance downgrade, which I am pretty confident is only a matter of time. Based on commentary so far, the downgrade will be a surprise and so $NEU will take a hit.
If NNZ-2591 results are not positive AND DAYBUE disappoints, I'd potentially exit quite quickly.
Looking to $NEU Strawman Bulls to challenge this ... after all its a strawman!
Disc: Held in RL and SM
As @Nnyck777 has highlighted, $ACAD announced their Q1 results including sales for DAYBUE.
I attended the call early this morning, and wanted to drill into the results in a little more detail. (all numbers USD)
While sales of $75.9m represented a decline of 13% over Q4, this had been signalled at the FY23 results presentation in February.
$ACAD has retained 2024 Guidance of $370-420m.
Decline, what are you talking about?
So, why the decline, if such strong growth is expected for the FY? Three reasons have been cited:
- Many patients who started in Q3 and Q4 faced admin delays with payers over the holiday season
- The initial surge of new patients in Q4 also saw a high level of discontinuation, as not everyone can tolerate the drug well vs. the benefits they see
- Severe weather during the East Coast winter saw access to prescribing physicans, especially, the important Centres of Excellence fall, due to a reduced number of "Rett Clinic Days"
$ACAD reported that prescribing has now picked back up, with the last 6 weeks showing postive net patient additions. Because of this they are holding to their guidance for the full year. The high level of discontinuations in the early part of the year as a result of the surge in prescriptions in Q4 has now levelled off.
My Revenue Analysis
The table below indicates the kind of quarterly revenues that will be required to hit the revenue guidance range of $370-$420 (midpoint $395)
Table 1: Daybue Sales Scenarios Required to Hit 2024 Guidance
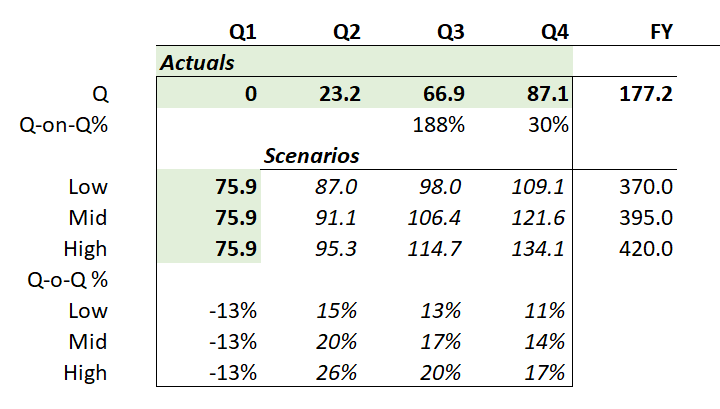
So how reasonable is this?
Well, at the FY call, $ACAD disclosed the following information, in terms of number of patients taking the drug:
- End Q3: about 800
- End Q4 about 900
- 27th Feb: 860
- 8th May; [862]
Today, I think I heard them say there are 862 patients on the drug (I'll have to check the transcript when it comes out as I am not sure I heard this correctly).
If that's true, and if 862 represents 6 weeks of net additions, then its likley that Q2 Revenue could reasonably get back to the low point or the mid-point of guidance. What we don't know if what the trough was and what the rate of recovery has been.
If they could get back to 900 patients by end of Q2, that would require net weekly addition of 5-6 patients. At that level, average patients for the Q would be about 870-880, compared with c. 850 in Q4. So, in broad terms, you'd expect Q2 revenue to be north of $87.
Importantly, with all this information and the "noise" of Jan and Feb out of the way, the Q2 result is going to be a key predictor of longer term growth. This is for three reasons: 1) the intial surge of highly motivated patients has passed, 2) we will be clear of the seasonal noise and 3) the product is now moving into a more steady state / linear growth phase.
Other Key Insights
To date, 1300 of a prescribed population of 5000 have started taking the drug.
Although it is believed that 6000-9000 people have the condition in the US, and it is expected that there will be increased diagnoses, we haven't heard any evidence of that, as yet.
9-month persistency is 58% (versus 47% on the Lavender trial), so the real world setting outperformance on persistency above the clinical trial appears to be holding up.
Importantly, $ACAD have reported that of the patients who took the drug during the Phase 3 trial, more than 50% are continuing to take the drug after 2 years. They further said that discontinuations are at a maximum over the first two refills, and decline significantly after refills 4 and 5.
On discontinuations, they have observed the following:
- Some patient discontinue before they have titrated to the effective dose and therefore may not be seeing the benefits
- Some patients prescribed outside of COEs, may be starting on the label dose, and then discontinuing due to GI side effects
- They are having success with GI management plans
Overall, as experience with the drug grows, they believe there is an improvement opportunity to help physicans and patients 1) have realistic expections 2) be patient to wait for benefits to show through and 3) improvement management of side effects.
International Timeline
We got some more specific details on the timeline:
- In Europe, they have engaged with the EMA, who has agreed the Pediatric Investigation Plan (PIP). They expect to file the NDA in Q125. (I think this indicates that a decision in 2025 is possible, depending on the priority given by the EMA)
- In Japan, they have scheduled a formal meeting with the PDMA to "discuss clinical plan"
- NDS (New Drug Submission) has been accepted for filing in Canada, and priority review granted. Decision is expected around end of 2024
My Conclusions
These are OK results and $ACAD appear to be managing the challenges that come from patient experience with the drug.
It is important to be realistic. Rett's is a very challenging condition for sufferers and their families. The drug does deliver some benefits, but not to everyone. And treatment brings unpleasant side effects to many. Depending on the individual patient experience of benefit versus discomfort, a significant proportion of patients abandon treatment.
It is encouraging that Canada have granted priority review. The decision in Canada later this year will be a key marker as to how a regulator assesses the drug with a longer body of clinical trial and real world experience to consider. The Canadian decision will be a key leading indicator for the EU and Japan.
From my reading of the report, the low to mid range of guidance remains on track. The upper end looks challenging and potentially out of reach. Where we end up will depend as much on how successful $ACAD are in their sales and marketing execution, particularly as they move beyond the COE's into the large institutions and small clinical practices.
I am less bullish about DAYBUE that I was at the end of last year. Today's result was less definitive than I had hoped, as it is not clear what the current trajectory is.
I am not sure how on top of the $ACAD details the Australian investment community will be, and it will also be interesting to hear how $NEU portrays the results.
In any event, much of my investment thesis rests on NNZ-2591, so I remain a HOLD. Today's result indicates that sales of DAYBUE are recovering from the early bumps in the road. But Q2 will be an important confirmation.
(Will post a brief update when I can access the presentation transcript)
Solid results on the Acadia earnings call. 1300 patients now on Daybue numbers picking up again the past 6 weeks. Progress into Canada by end of 24 discussions with Japan commenced. Eu possibly early 25. Children remaining on 75-80% of titratied dose.
Standard of care plans in development to manage GI issues.
$74 million qrt in line with expectations.
On Livewire today: Backed by industry titans, this fundie is leveraging Australia's healthcare innovation
also includes a related video interview (of Hashan De Silva - KP Rx Healthcare Opportunities Fund) on Youtube: https://www.youtube.com/watch?v=BBXWHkI72UQ
both of which talk to Australia's innovative healthcare companies in general (with comparisons to the US) and speaks about NEU in particular
Disc: Held in RL & SM
$NEU have announced this morning that Health Canada has granted Priority Review status to the NDA for trofinetide.
The core data supporting the application is the Phase 3 LAVENDER study.
The announcement is significant for several reasons:
- It confirms that $ACAD initiated the application on time with their Q1 2024 target
- It means that with a target review time of 180 calendar days for Priority Applications (compared with 30 caledar days for Standard Reviews) , they will likely achieve their indicated milestone of a decision in Canada before YE 2024, opening an incremental market opportunity of 600-900 patients, which would be expected to show strong pent-up demand given the community response across the border
- As the first regulatory agency after the FDA to signal priority treatment, it bodes well for approaches in 2025 to the EU and UK, although note that $ACAD lacks a presence in these markets.
Overall, an incremental positive step.
Disc: Held in RL and SM
Ultragenyx has just reported on its successful completion of its past 1/2 Angleman Syndrome trial.
“GTX-102 is an antisense oligonucleotide developed by GeneTx, designed to inhibit the UBE3A-AS DNA strand.”
The patients in the trial showed a significant improvement across several areas on the Bailey-4 development scale scores. These improvements were greater for the children on the drugs compared to the natural development scores of children with Angelman who are not on treatment.
Ultragenyx still has not had its phase 3 meeting with the FDA. There are some safety concerns sighted with several patients developing lower extremity weakness, which appeared to be reversible on sensation of treatment.
This is the major competitor to NEU. The drug work by targeting DNA - a very different therapeutic pathway. Even if both are succesful they may be used in conjunction.
It will be interesting to see if NEU also uses the Bailey-4 scale for its primary end points. This will be different to the scoring used in the Phelan Mcdermid study.
The Phelan Mcdermid study used:
o ClinicalGlobalImpressionofImprovement(CGI-I)-scale
o CaregiverOverallImpressionofChange(CIC)–scale
I would say it is unlikely that Neu would swap mid study which means the two drug programs Neuren’s and Ultragenyx may be using different measurement scales. It will be interesting to see whether the FDA looks for a more consistent - gold standard measure for the future Phase 3 trials.
https://endpts.com/ultragenyx-preps-for-pivotal-angelman-syndrome-trial-aan24/
Snapshots of Phase 3 progress of Trofinetide for Retts syndrome
Took nearly 3 years for Phase 3 trial to finally complete (Oct 2019 - Feb 2022)
Have done this to understand the timeframe for Phase 3 trials given I'm holding a couple of bios currently undergoing phase 3 at the moment.
24 Feb 2023

25 Feb 2022

24 feb 2021

6 apr 2020

Oct 2019

Phase 3 commencement announcement 31 Oct 2019

[held[
$ACAD presented on 18 March at the UBS Virtual CNS day. There were two bits of interesting information about the ongoing progress of DAYBUE.
- Persistency continues at about 10% above the level in the clinical trials. While not an increase, it is good that this level is holding steady. In the Q&A, the reasons for increased persistence are 1) in RL physicans are titrating up to manage tolerability and 2) in RL there is proactive applicatoin of GI management plans, whereas GI management wasn't applied until quite late the in the CT.
- The market is expanding. CEO Stephen Davis stated that at launch, in 1H 2023, there were 4500 diagnosed Retts patients in a market where the calculated prevalence is 6000-9000 (US only). According to $ACAD, the number is now 5000, an 11% increase in one year. He went on to say that this is a common observation in rare untreatable diseases, implying that one a treatment becomes available, there is an increased propensity to make a diagnosis of the condition.
Analysts tried to drill into the reported weather-related (clinic closures) in January, which reduced sales in January, with recovery in February and March. Clearly, the analysts (aware of the short thesis) are trying to understand whether there is any slowdown in sales and have been talking directly to the CoE clinics.
Nothing in the presentation indicates that $ACAD are changing their view on 2024 revenue guidance, although it wasn't discussed explicitly.
$ACAD are scheduled to report 1Q 2024 results on 30 April. I'm expecting a softer DAYBUE sales number than Q4 23, and the market reaction will depend upon how closely $NEU holders and analysts have been paying attention, although CEO Jon has been clear to communicate that 1Q 24 revenue will be lower than 4Q 23 as a result.
Disc: $NEU held in RL and SM
An excellent summary of where Neuren stands and potential acquisition value. See below
https://www.livewiremarkets.com/wires/neuren-where-to-from-here-2024-03-23
I've been trying to think why $NEU price plunged 15% yesterday on them releasing the DAYBUE sales results.
My hpothesis is that when the market saw the chart below from the release, they thought this was showing TOTAL revenue. Its not, Its showing Royalty Revenue, as labelled in the slide.

They didn't include the US$50m milestone payment. Today we see a more complete view, below.

Were ASX investors so jumpy about the short report that there was a knee-jerk reaction to seeing an incomplete number below consensus? Can investors be so dumb? Or am I missing something?
Whatever, I increased my RL position by around 25% at the dip. Yay. (And upped on SM, at a slightly less good price)
Just on the results call now.
Acadia Pharmaceuticals ($ACAD) have announced their Q4 results.
Sales of Daybue came in at $87.1m.
This compares with $66.9m in Q3, and is at the top end of the guidance range $80.0 to $87.5m.
$ACAD have set FY24 guidance for Daybue at $370-$420.
In terms of implications for $NEU, the targeted sales will trigger the $50m milestone for sales >$250m, but it looks like the next milestone payment of $50m will fall in FY25.
Considering the 4Q run-rate of $87.1m as $348.4 annually, the FY24 guidance represents growth over the 4Q run rate of 6% to 21%, which means that $ACAD still see growth ahead.
The recent Culper Research short report claimed that Daybue sales peaked in Q3, so I think the short thesis is severly undermined by this result.
Just going on the call now, to see what further colour there is on the result.
Disc: I hold $NEU in RL and SM
When you are heavily invested in a stock and a short report is released targeting your major revenue stream or investments it is not a nice feeling. Many of us Strawpeople would have experienced this before. One example was February 2021, when J Capital Research released its short report on Nearmap. Thankfully this report was easily refuted. The company's share price recovered quickly.
Thank you @mikebrisy for linking this short report see here (Culper Research Short Report). Great summary @Slideup.
Perhaps it is best to read this document from back to front. I think a short report is an excellent opportunity to pay attention to your investment thesis. I decided to take a few hours to go through the report, FDA website and latest research, as well as follow the money and really think about my position. I will have confirmation bias which I am trying to logic my way through and full disclosure I took this 15% drop on Friday as an opportunity to buy a few more shares in my already overweight position in Neuren Pharmaceuticals.
My immediate thoughts that lead to this purchase were:
1. I know this stock, the drug works, I have done my due diligence (DD), I follow social media accounts and I am seeing the positive effects of the drug in real time.
2. The science works and Acadia plus other unnamed pharmaceutical companies were in a race to buy the rest of world rights to Daybue and they all would have done their DD
3. Neuren has a second drug that is likely much more valuable than Daybue and has had recent outstanding phase 2 results for its trial in Phelan McDermid patients. The drug also has fewer side effects than Daybue
4. There are 3 more impending phase 2 trial results for Neuren’s second drug which will act as short term catalysts
5. I know that there a sharks circling who may use unscrupulous methods to absorb more Neuren or Acadia stock or apply pressure on the share price as we know Neuren is a current acquisition target
The Culper short report targeting Acadia Pharmaceuticals released on friday was unexpected. I am curious whether Acadia or its licencing partner Neuren was specifically the target of this report. It is possible that Culper may have had an incentive to quickly wind up a potential short position in Acadia. A quick short and distort attack can induce panic selling and allow a shorter to quickly get out before they are caught in a financial disaster.
Acadia had two positive updates recently:
1) Acadia recently won its patent litigation for Nuplazid - giving the company further protection from generic competition into the future and preserving revenue growth of the drug.
2) On the 9th of January 2024 Acadia reported an increase in guidance for Q4 from $80 million to $87.5 million for its sales of its newest drug Daybue.
Given the above points, anyone with a short position was likely to be in trouble come the February 28th, 2024 when Acadia released its financials. The share price would likely respond positively to this upcoming announcement.
The 8% drop following this reports release could have been an exit strategy or perhaps Culper really has increased its short position. However US analysts covering Acadia obviously didn’t think the report held much water as there was a full share price recovery on the same day.
The Report
The Culper report is full of hyperbole and emotive language – examples in the report assert that Daybue has been a total flop.
The report uses phrases such as horror stories to describe the stories spreading through the Rett community regarding Daybue.
The report also takes aim and Acadia’s credibility itself suggesting a downplaying of adverse events by the company and that 1 in every 10 to 11 patients who use Daybue end up in hospital.
Firstly, a total ‘flop’ of a drug doesn’t see such fast uptake and increasing revenue. Nor does a pharmaceutical company who is expecting sharp declines in uptake and increases in discontinuations pay such a hefty premium for the rest of the world rights for a drug.
With regard to the FDA Adverse Event Reporting System or (FAERS), this is a public post-drug release monitoring system. This database does not analyse the cause of hospitalisations or separate the cause from the drug or the disease.
Daybue is not a cure for Rett and ongoing health conditions and vulnerabilities from the disease will continue. There is no comment or comparison of standard hospitalisation rates for patients with Rett Syndrome versus hospitalisation rates for Rett patients on Daybue in the report. Acadia has been very clear that side effects and dosing management and assistance is a crucial part of successful treatment while on Daybue. It advised all patients to start on low dosing levels and titrate up as side-effects are managed.
FDA Adverse Event Reporting System (FAERS)
The FDA website states:
“Most common adverse reactions, occurring in at least 10% of Daybue-treated patients and twice the rate of placebo, included diarrhea (81%) and vomiting (27%).” See here (FDA reported Daybue Side Effects)
Culper report alternatively states “nearly everyone” experienced adverse events on Daybue. My interpretation of Culper’s statement is that the research house may be taking the total number of reported adverse events in the FDA database, which is 1115 cases and ascribing this as if everyone on Daybue has reported an adverse event. 1005 reports of diarrhea were made in the database and that is likely where they are falsely arriving at the >90% prevalence of diarrhea amongst those taking Daybue.
However as seen below the FAERS does not work like that. Reports are not verified, multiple reports may be in the system and causation cannot be linked to the drug. This is clearly stated on the FDA website.
Furthermore there was no black box label assigned to Daybue. This is given to drugs:
‘’when serious adverse reactions or special problems occur, particularly those that may lead to death or serious injury.” (according to the FDA website)
Furthermore the FAERS database has the following statements attached:
1. Duplicate and incomplete reports are in the system: There are many instances of duplicative reports and some reports do not contain all the necessary information.
2. Existence of a report does not establish causation: For any given report, there is no certainty that a suspected drug caused the event. While consumers and healthcare professionals are encouraged to report adverse events, the event may have been related to the underlying disease being treated, or caused by some other drug being taken concurrently, or occurred for other reasons. The information in these reports reflects only the reporter's observations and opinions.
3. Information in reports has not been verified: Submission of a report does not mean that the information included in it has been medically confirmed nor it is an admission from the reporter that the drug caused or contributed the event.
4. Rates of occurrence cannot be established with reports: The information in these reports cannot be used to estimate the incidence (occurrence rates) of the events reported.
Patients should talk to their doctor before stopping or changing how they take their medications.
The number of adverse events reported by the public for 2023 and 2024 are reported below in Figure 1. Remember these are not causative, there may be duplicate reports and reports have not been verified. 971 events were attributed to 2023 and 144 to 2024 so far.

Figure 1: FDA screen shot from FAERS database ( Source see here: FDA DAYBUE REPORT NUMBER IN FAERS)

Figure 2: FDA screen shot from FAERS database by reaction type reported by the public (see here FDA DAYBUE REPORTED REACTION TYPE)
As you can see the main reported % events are in green which represents non-serious events. Hospitalisations are a minority of the events recorded. Gastro intestinal disorders are by far the most common non-serious event reported which matches the Lilac study expectations see Figure 3 below.

Figure 3: FDA screen shot the most commonly reported non-serious Gastro-Intestinal events.
The next most common adverse events reported were issues such as under-dosing, with 494 events reported. See Figure 4 below. This matches the initial suggestions made by Acadia to start on a lower dose and titrate up as patients began their treatment on Daybue during 2023.

Figure 4. FDA screenshot of other most common adverse reporting on FAERS relates to dosing.
We can draw no causative conclusions from FAERS but no life-threatening events have been reported. It was made clear by Acadia and Neuren that Daybue had side-effects and many community support groups offer assistance with this.
A 2023 Epidemiology report paper released in the US offers some better insights into what the average Rett patient experiences. This would have been published just prior to patients receiving Daybue. (see study here).
The authors acknowledge gaps in available data for Rett patients in the US. Highlighting a lack of understanding around the following:
· Health care resource utilization and
· Health care costs
Of the Rett patients surveyed nearly 25% had hospital admissions for lower respiratory tract infections in the previous 5 years. Roughly 12% had 2 or more admissions. Unfortunately, there were no % rates for overall hospital admissions given in the study.
It is difficult to determine then if 1 in 10 or 11 Daybue patients being hospitalised is more or less than the number of hospitalisations expected by Rett complications alone. So the quote in the Culper research report offers no particular insight as there is no context whether Daybue causes higher hospitalisation rates.
The most common clinical manifestations of Rett without Daybue treatment include:
· Neurological disorders 72.8%
· Gastrointestinal/ nutritional disorders 41.9%
· Orthopedic disorders 34.6%
The authors reported an average of 44.43 health related visits per patient per year. Feeding assistance (37.9%) was the most prevalent supportive therapy required for Rett patients. So gastro-intestinal and nutritional management is definitely part of the Rett treatment pathway whether on Daybue or not. The requirement for anti-epileptic drugs (54.8%) was also a prevalent support requirement. The conclusion of the above study was that Rett patients had a substantial disease burden. The FDA adverse events reported in the short report very much match the disease burden of a patient with Rett syndrome.
Gene Therapy
Another example of emotive writing in the report is the title used in the Gene Therapy section. This title eludes to new gene therapies being a potential “harbinger” for Acadia.
Let’s explore:
I agree with the report that there is a limited number of patients with Rett Syndrome. Hence there is a small population of potential trial subjects to pull from. Gene therapy has been mentioned by Jon Pilcher, Neuren CEO, as a possible treatment, not cure, for Rett Syndrome but a final treatment is years away, if successful at all. It is also something that could be used in conjunction with Daybue so patients can have multiple treatment options.
Taysha Gene Therapies is currently conducting phase 1 / 2 trials for its TSHA-102 drug in adults with Rett Syndrome. This gene therapy is delivered via injection into the intrathecal space see Image 1 below. This drug regulates MECP2 expression.

Image 1: Injection into Subarachnoid space or lumbar areas are the most common delivery pathways for gene therapies. Source (here)
This is obviously a more complicated treatment pathway than an oral dosing in Daybue. The treatment is currently only in adults with Rett’s and the company only plans to run the phase 1/2 REVEAL trial on 2-3 adults.
Forty-two days following the initial trial the first patient results were released (Taysha Gene Therapy Initial Trial Results). Results on 1 patient were as follows:
4 weeks in:
“Four weeks following the administration of TSHA-102, the patient demonstrated a score of 2 (“much improved”) on a version of the Clinical Global Impressions–Improvement scale adapted to Rett syndrome, an improvement to a score of 5 (“markedly ill”) from a baseline score of 6 (“severely ill”) on the Clinical Global Impressions–Severity scale, and an improvement to a score of 29 from a baseline score of 52 on the Rett Syndrome Behavior Questionnaire. Conversely, on the Revised Motor Behavior Assessment, there were no marked changes at 4 weeks after dosing.”
6 weeks in:
“At 6 weeks after administration of the gene therapy, observations of improvement in autonomic function, sleep quality and duration, normalization of night time behavior, and social interest were reported. Gained abilities to sit unassisted for 3 minutes, to hold an object, to unclasp hands, and to use fingers to touch a screen were also noted. At 5 weeks after treatment with TSHA-102, there had been no quantifiable seizure events recorded. “
Study summarized
“Following treatment, we have observed improvements in breathing patterns, vocalization, and motor skills. The patient was able to sit unassisted for the first time in over a decade, and she demonstrated the ability to unclasp her hands and hold an object steadily for the first time since infancy.”
TSHA-102 was well tolerated in the 1 patient it was trialled on and there were no significant adverse events reported. The second patient is now in the trial. FDA has recently approved a new drug application to commence treatment trials on children in the US. Taysha is also looking to run a trial on children in the UK.
There will be patients who may struggle or discontinue Daybue who are keen to be involved in a Gene therapy study. However, gene therapy is an unproven treatment that is years away if it proves successful at all. Patients who are seeing improvements will be unlikely to cease Daybue for an unproven treatment. Companies such as Taysha Gene Therapies are likely to struggle to recruit patients as there is an existing FDA treatment available for families with Rett children. There are still potential adverse events of gene therapies that are still unknown.
The short report describes Daybue’s many “short-comings” and suggests that gene therapy approaches are compelling.
The difficulty with the statements made by Physician’s quoted in the short report is that the testaments of these doctors largely make up the basis for the justification of Daybue being a flop. In Culper’s very own disclaimer -they acknowledge that these statements may not be fully truthful and are potentially bias and may leave out positive statements. Furthermore doctors were often financially compensated for their statements which they acknowledge might have skewed their report.
On the 10th of January this year Taysha announced that it was initiating the REVEAL paediatric trial in the US. TSHA-102 was expanded to female patients 5-8 years old. This study would include US, UK and Canada and the first trial was to include 3 children. This would test safety and efficacy of Taysha’s gene therapy in a paediatric population. The results for this study are due mid-2024. See announcement (here)
The dose is a single lumbar intrathecal injection. The studies so far are recruiting very small numbers of Rett patients with n=6. This is hardly going to lead to mass cessation of Daybue by Rett patients in order to participate.
Clinical Trials.gov website shows that the TSHA-102 Phase 1/2 Trials won't be completed until:
Estimated Primary Completion Date :
November 2, 2028
Estimated Study Completion Date :
November 2, 2031
Source (here).
Neurogene Trials
The second gene therapy trial in Rett syndrome also targets mutation in MECP2 gene like Taysha. The MECP2 protein regulates the activity of genes critical in brain development and function. The NGN-401 therapy delivers a working MECP2 gene. The thought is that a functional gene will lead to normal regulation of activity by the MECP2 protein. It is delivered via an adeno-associated virus (AAV) to deliver the gene inside working cells.
Phase 1/2 clinical trials begun December 2023. Two girls are being treated and it so far is well tolerated. A third patient begins treatment at the beginning of 2024. Efficacy data readouts will be available at the end of 2024. N=5 and girls are between 4-10 years of age. So another very small study.
There is currently no follow up data available. If the study continues and there are no serious adverse events further recruitment will begin in the second half of 2025. Source (here).
Further Information on the NGN-401 study can be found on Clinical Trials.gov (here) according to this website:
“The study is a phase 1/2, open-label study designed to assess the safety, tolerability, and efficacy of administration of an adeno-associated viral vector serotype 9 (AAV9), using Neurogene's proprietary transgene regulation technology. NGN-401 contains a full-length human MECP2 gene which is designed to express therapeutic levels of the MECP2 protein while avoiding overexpression.
The study treatment will be administered under general anesthesia via intracerebroventricular (ICV) delivery. Each participant will be followed for safety and preliminary efficacy for 5 years after treatment and is expected to enroll in a long-term follow-up study for 10 years.”
According to Clinical Trials.gov website Phase 1-2 safety will take up to 10 years to establish which provides a wide moat for Daybue as the only safe and efficacious treatment of Rett.
As Taysha’s gene therapy is a similar process I imagine this is why Jon has been so unphased during question time on Neuren calls as possible phase III trials may be a decade away.
The lead time for Daybue seems to be longer than I was actually modelling for. A quick google search reveals these time frames are hardly a harbinger for Acadia as there will still be years before a phase III trial, if that is ever reached.
Funding Taysha Gene Therapy
RA capital management is funding Taysha’s Gene therapies to the tune of $150 million.
About RA capital management:
Assets under management: 9.65 billion USD (March 2023)
Founded: January 2002
Founders: Richard Aldrich; Peter Kolchinsky
Headquarters: Berkeley Building, Boston, Massachusetts, U.S
Interestingly RA capital which is funding Taysha Gene Therapies also had a large stake in Acadia Pharmaceuticals in 2014.

Funding Acadia
There are 573 institutional owners and shareholders.
Shares on issue: 169,300,389
Largest shareholders: Baker Bros (26%). Vanguard, Black Rock, State Street Corp etc…

I can’t see whether RA capital still holds any Acadia stock. However in 2016 an Acadia Shareholder suit ended in $15.75 M settlement against RA Capital Management LCC claiming that they colluded with two investors to score millions in short-swing profits from the drug company in violation of the securities laws (source (here)
The same RA Capital and Peter Kolchinsky that is funding Taysha Gene Therapies.

Further to this A Fierce Biotech article (here). States that RA Capital is hardly pristine. And has a history of settling with the US Securities and Exchange Commission. In another case they were ordered to pay US$3.6 million in disgorgement, interest and penalties related to 17 instances between 2009-2013, where they bought shares in stock too soon after selling short the same stock.
“This allows a fund to buy shares in a company offering at a lower-than-market price to cover its short position. This episode highlights a fundamental tension that plagues life science hedge funds between supporting innovative companies and deriving financial benefit--regardless of whether it's to the detriment of those same companies.”
Funding Neurogene pharmaceuticals
Neurogene is a fully owned subsidiary of Neoleukin (Source: here). The merger was completed in December 2023.
“Neoleukin is focused on developing immunotherapies to treat cancer, inflammation and autoimmunity, leveraging its de novo protein design technology.”
Investors include: Casdin Capital, EcoR1 Capital, Great Point Partners, Janus Henderson Investors, Redmile Group and Samsara BioCapital were among those taking part in the financing round
Rachel McMinn is Neurogene CEO and founder.
Culper Report Insurance Payment Issues
Insurance payment issues for Daybue are certainly a valid concern for all investors in Acadia and Neuren on page 11 of the report Culper suggests that Acadia is now facing a wall of discontinuations. They suggest 6/12 insurance re-authorizations are likely to be rejected based on minimal improvements by patients who have been on Daybue.
Page 13 of the short report discusses issues with insurance plans. This is true of all drugs in the US system and the PBS system in Australia too. To stay on an expensive drug patients have to achieve improvement to justify ongoing costs. This area is challenging for patients to navigate. Acadia has set up help lines to help patient streamline payments from insurers.
I acknowledge this is a risk but I trust that Acadia has a strategy to assist patients navigate these difficulties. Future qrtr growth beyond this 6 month window should certainly help to alleviate investor concerns.
According to the report numerous insurance plans require an improvement of CGI-I scores of 1 to 3. There is an argument that due to the lower dosing and slower titration up, patients may not achieve the required improvements within 6 months to receive continued payment.
It appears in the report that physicians have complaints of time management when filling out these tests for patients. This is certainly a common complaint across all areas of medicine and is not unique to Daybue.
Caregivers indicated in the report that they are facing challenges of navigating a complex system. However this once again is not unique for Daybue.
The report also claims that patients are losing interest in Daybue due to its side-effects. On page 10 of the report Culper sites reduced Facebook engagements and impressions since Daybue’s release in June 2023.
This trend may be falsely attribute to drop out rates and alternatively may be caused by the opposite effect – less need to post as more and more caregivers have started Daybue and are managing the side effects so they are less likely to post for advice. High initial post rates may also have been related to questions about Daybue applications and insurance coverage.
There are 5 physician quotes in the report sighting a variety of reasons patients are reluctant to start Daybue e.g. due to diarrhea. It is difficult to put much weight in this anecdotal evidence when a sample of 5 other physicians are likely to give different answers again.
There are certainly common stories of caregivers having to titrate Daybue dosages up and down and find dietary solutions to help with symptoms before further increases. This is true. Is this leading to attrition? Certainly some but so far Acadia denies high rates of drug attrition and claims a 76% retention rate at 6 months. Culper questions this and states that churn is likely “closer to double that.”
This assumption of double the churn appears to be based on the statement made by a Physician interviewed for the report who suggested roughly 40% of patients cannot safely reach their full prescribed dose without safety issues.
I do acknowledge that this is a potential risk of Daybue. However I await further confirmation of these numbers directly from Acadia.
Financials
The Culper short report proposes that the market analysis is incorrect in its assumptions regarding the financial projections of Daybue and that sales have already peaked in 2023 and are going to fall moving forward.
They double the churn reported by Acadia and a much higher attrition rate due to side effects and insurer payee issues. They also sight gene therapy as a major threat to Daybue usage as patients will opt to cease this medication in search of access to gene therapy trials.
As explained above no gene therapy trials are likely to begin phase 3 which will require more participants than phase 1/2 until 2028-2030. So this is toward the very end of the proposed model. The current trials require very small sample sizes until safety and efficacy is established. Gene overexpression is a risk that requires serious caution especially in children.
Culper Research history
At the beginning of this short report there is a disclaimer that I suggest everyone reads (Culper Research Short Report).
The disclaimer basically states that they are not liable for any losses from the information provided. That you should assume they have a position or are affiliated with people who have a position in the securities discussed in the short report. That Culper is or affiliates are long, short or neutral on the stock discussed in the short report.
There is a statement that they are continuing to transact in the securities that there is no warranty around the information provided and that they cannot attest to the accuracy of statements provided and quoted. That positive comments may not have been included and that the people quoted may have received compensation which could make them bias representatives.
Culper involved in previous defamation
Life MD Inc Vs Culper– involved in defamation suit
Culper Research and owner Christian Lamarko allegedly defamed Life MD Inc, CEO and CTO and claimed the pair had engaged in fraud in a previous company and had engaged unlicenced doctors to dispense medications.
See news article ( here)
Life MD Inc was a telehealth company offering prescription based products and services for areas such as dermatology and hair loss. On April 14 2021 Culper research published a short report on the company.
Defamation proceedings were bought against Culper Research and Lamarco shortly after this report was released. See defamation suit here.
Life MD CEO and CTO claimed that the report was a short and distort attack:
A “short and distort” stock scheme occurs when “short-sellers borrow securities, sell them, and then drive the price of their target company's stock down by spreading materially false, misleading, defamatory, and disparaging information about the company. Once the company's stock drops to an artificially low price, the short-sellers repurchase and return the borrowed securities, pocketing the difference.”
Side note: Interestingly in this defamation case of Life MD Inc vs Culper Research and Christian Lamarco a footnote states:
“Christian Matthew Lamarco is the sole employee and member of Culper Research.”
This is apparently a very small research house of one.
The case is ongoing.
Summary
This short report has given me a chance to catch up on the latest gene therapy trials and investigate the FAERS database for myself. I am not currently overly concerned and I never believed or modelled a 100% retention rate or 100% TAM in the Rett community for Daybue.
Daybue has side effects and they are manageable the benefit of this drug is life changing and worth the management of side effects. I will continue to hold my position and my conviction remains unchanged. Although I acknowledge potentially lower than modelled revenue for 2030 If attrition rates are slightly higher than expected.
I have read and been thinking about the Culper research short report targeted at Arcadia and indirectly Neuren. Of the short reports that I have previously seen I consider this to be one of the lower quality attacks. It is very opportunistic timing 10 days before ARCADIA release their quarter numbers. Neuren's SP has also doubled recently so people are more likely to sell to lock in a gain, so this could be an attempt to establish a long position in Neu but thats neither here nor there. I did invest more in Neu yesterday after the trading halt as I am comfortable with the data that I have seen and I think Jon Pilcher is an honest CEO and I would be surprised if they were flogging a dodgy drug.
The short report is very heavy on emotive wording, lacks verifiable information and generally uses strawman (not the good platform type) arguments to support its points. It uses a nice trick of generating authority by attributing quotes to authority sources (Dr's, or de-identified facebook patient quotes etc) that are unidentifiable so their validity can not be verified. The disclaimer at the front of the report is also worth a read too and basically tells you that they will make stuff up.
The four main arguments of the short report are:
1: The treatment (Trofinitide) is ineffective, poorly tolerated and is causing significant adverse events
2: Patients are either not continuing its use or can't get insurance re-embursement so patient numbers have peaked
3: Insiders at ACADIA are leaving
4: Gene therapy treatments will become available and take the market
These arguments taken at face value and read in the emotive language of the report are pretty concerning. It also plays nicely into the the recency bias --> where the most recent piece of information we have is given more weight than older information. The trick here is that the brain isn't very good at separating real from fake information.
The 1st argument that the treatment is ineffective has some merit to it as we have known from the development trials that not all Rhetts patients would benefit from or tolerate the drug. Notice the link they provide goes to the prescribing medicine information rather than the peer reviewed study data which breaks these adverse events down.
Culpers wording:
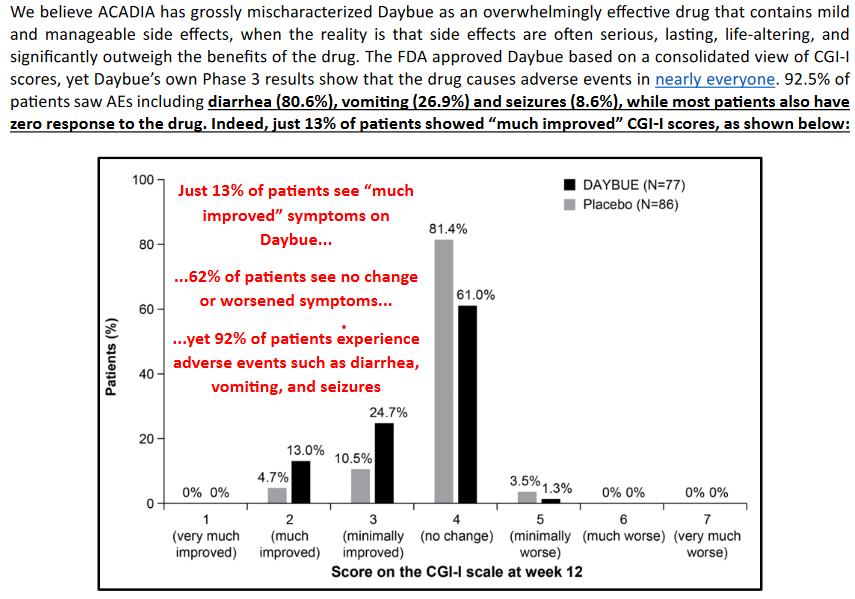
This information while true is misleading as this data is from the intial 12-week double blind trial. Nothing like quoted percentages in red ink to cause panic. The design of the study with good descriptions of the response metrics and the actual results is worth reading. Importantly an adverse effect in itself does not necessarily mean much. As part of these studies everything is reported and can be unrelated to the drug itself. In the Lavender study 2% (n=2) of placebo patients had adverse effects leading to drug withdrawal vs 17.2% (n=16) in the trofinitide group. Diarrhea and vomiting were the main adverse impacts and table 2 breaks down the severity of these effects with most being mild to moderate. Its worth pointing out that since this trial Acadia have developed treatment plans to manage these effects and there is good information about how they are doing this. Despite this diarrhea is still the main adverse event. ARCADIA are still fine tuning patient management of this event, and it is a valid risk that the severity of these issue limit the long term use or total dose rate. But for now this information is not conclusive.
Importantly, and what the Culper research failed to mention is that this initial 12-week Lavender trial transistioned into a 40 week open label trial called Lilac 1, where any patients who wished to continue the treatment could, as well as any patients on the placebo could start on trofinitide. 90% of the patients on Trofinitide in the Lavender trial continued using it. After the 52 weeks (Lavender + Lillac-1 time) only 54% (84/154) patients continued on trofinitide, with the majority dropping out due to adverse events (probably diarrhea), and 3% discontinued due to lack of efficacy.
77 of these patients then transistioned into Lillac 2 whose aim was long term safety and efficacy data. It ran for 32 months. 79% (n=60) of patients completed the study. In this study a further 3.9% (n=3) discontinued due to lack of efficacy and their were 5 deaths but these were due to causes unrelated to Trofinitide. Some patients have now been on the drug for 2.5 years. So this gives a reasonable amount of confidence that trofinitide is relatively safe and patients do see a benefit from using it. Diarrhea is still an issue as the main adverse event. This data was presented in December 2023 and a short video update was also provided by Dr Ponni Subblan - Acadia chief medical officer. The efficacy data over this period is consistent with the Lavender study, with most patients having minimally improved CGI-I scores. I am taking the Dr's interpretation on the CGI-I and the RSBQ scores and believe the benefits that they have published in peer reviewed journals. I think that the continued use of Trofinitide by patients and the willingness to tolerate the diarrhea will probably come down to the perceived improvement from the carer. So I think the best way to read this is to watch the ACADIA quarterly numbers.
From the data above I cant see how a "wall of discontinuation is about to hit ACADIA". Likewise, the argument Culper make that ARCADIA are misrepresenting the true discontinuation rate also doesn't make much sense to me. I don't think it matters if the patients tell their Dr that they are stopping or not as the uptake/discontinuation rate will be monitored through sales of Trofinitide, which ARCADIA would have as an early read and I can't see why ARCADIA would inflate this. Given they have guided for sales of between $80-87M, anything below this will be a concern and anything below the $66M they achieved last quarter would be an immediate sell and would validate Culpers patient discontinuation argument.
The insiders leaving ARCADIA argument doesn't really hold much weight - 3 senior people are leaving Doug Williamson Executive Vice President, Head of R&D (Jan 2023 - transition out currently) and Katie Bishop, Chief Scientific Officer (Jan 2021-Dec 2023). Arcadia has >500 employees, so we would expect some turnover and without anything but circumstantial conjecture I can't really see this claim being anything but a misdirection to make the situation seem dire. Especially since one of the recent hires is Kimberly Manhard as a Senior Vice President , Global Strategic Planning and Execution. Seems to me if the internal data on Trofinitide was dodgy you wouldn't be continuing to develop a global rollout strategy. Instead you would have raised capital after the last quarters numbers and projections before the "theoretical 6 month wall of discontinuations" hit you.
The gene therapy argument is not new and it is a genuine risk, but I don't think it will be a deal breaker for Trofinitide. If patients gain a benefit from Trofinitide then they will keep using it, likewise I am expecting that Arcadia are continually improving the management of diarrhea symptoms to make it more tolerable. The benefits and tolerability will determine whether ARCADIA's guidance is correct. Trofinitide has a significant first treatment advantage, currently there are no other FDA approved treatments. The gene thereapy enrollment trial they highlight is a phase 1 trial, so a long way to go before it is ready for the market. Gene therapy has been discussed on Strawman previously and it could be something great, but it is still a long way off so I don't really think at this stage it is a viable threat to Trofinitide.
I am also more comfortable buying into Neuren on this weakness due to the valuation being modest - although this is based on the trofinitide rollout continuing, and the recent positive readout from their NNZ-2591 phase 2 trial for Phelan-McDermid syndrome in December. I had previously prescribed this drug no value but after the phase 2 result I think this is now worth owning an option on it being successful.
That was a long winded way for me to say I think the Culper research was scaremongering.
Short Report on Acadia and Daybue.
$NEU SP plunges 17% on open.
It will be interesting to see how $ACAD responds in its results report next week.
I have a full position on $NEU, so won't be buying more on this opportunity.
Interesting. Short reports provide entertainment value, but sometimes there can be something in it.
Disc: Held RL and SM
Culper Research discloses short position in Acadia Pharma
February 15, 2024 at 12:26 pm EST
Share
Feb 15 (Reuters) - Culper Research has taken a short position on Acadia Pharmaceuticals' stock, it said on Thursday, sending the drugmaker's shares down as much as 8%.
Acadia's drug, trofinetide, to treat Rett syndrome, a genetic brain disorder, was launched in the U.S. last year under the brand name Daybue.
"Acadia has misrepresented Daybue's safety profile, and in turn, patent retention rates," Culper Research alleged in its report.
"The company now faces a wall of discontinuations due to insurance reauthorization requirements. Numerous insurance plans we reviewed require tangible proof of improvement on the drug," the report said.
Reuters could not immediately verify the short seller's allegations.
San Diego California-based Acadia, which licensed trofinetide from Australian drugmaker Neuron Pharmaceuticals , did not immediately respond to a request for comment.
The approval had allowed use of the drug in adult and pediatric patients two years of age and older, with a label warning of diarrhea and weight loss.
Culper the drug "will continue to decline over time as patients discontinue" treatment and the company "runs out of new patients to fill the gap" and estimates revenue from Daybue will be $316 million in 2024.
Analysts were expecting revenue of about $379 million for the drug in 2024, as per LSEG data.
Acadia has an antipsychotic drug sold under the brand name Nuplazid approved for patients with a type of Parkinson's disease.
The company's shares clawed back some lost ground and were last down 2.1%.
Short sellers make money by betting that the price of a security (such as a stock) will decrease.
(Reporting by Pratik Jain in Bengaluru; Editing by Shilpi Majumdar)
$NEU have just issued their quarterly report.
All the key news flow has been covered by releases, so I'll not rehash any of that.
The end-2023 balance sheet is strong with $229m, ample to support the clinical program for NNZ-2591.
In terms on ongoing revenue, Trofinetide is the driver, with the chart below showing the relationship to sales by $ACAD.
Just to clarify, we don't yet know the Q4 revenue number because $ACAD doesn't report Q4 until 25th Feb. That's a key date to watch, as the DAYBUE sales will provide a key datapoint on sale trajectory. My key question is: was the Q2 to Q3 jump pent up demand that will moderate quickly, or is the Q4 guidance conservative, in which case there could be an upside surprise?
So look out for a market announcement by $NEU at the open on $26th Feb unless, of course, $ACAD gives a sales update ahead of the earning release - which is possible - in which case $NEU would follow at the next open.
Last year $NEU reported FY results on 24th Feb, and I don't know what the plan is this year. Ideally, their FY result need to include the $ACAD 4Q revenue number.
Has John said anything about this before?
Beyond that, I can't see anything of particular note in the release. ($ACAD are still holding on to the PRV.)

Daybue’s main competitor for the treatment of Rett’s has failed to meet its primary end points.
This provides further Moat and validation of current financial models for Daybue.
Anavex Life Sciences Provides an Update on Rett Syndrome Program
Anavex Announces Topline Results from Phase 2/3 EXCELLENCE Clinical Study in Pediatric Rett Syndrome
NEW YORK – January 2, 2024
https://www.anavex.com/post/anavex-life-sciences-provides-update-rett-syndrome-program
Just saw a number of upgrades published this morning, here are the highlights from CMC:
19/12/20238:44AM Dow JonesNeuren Pharmaceuticals Price Target Raised 54% to A$27.00/Share by Bell Potter (headline only)
19/12/20238:44AM Dow JonesNeuren Pharmaceuticals Upgraded to Buy from Hold by Bell Potter (headline only)
19/12/20238:39AM Dow JonesNeuren Pharmaceuticals Price Target Raised 27% to A$31.10/Share by Petra Capital (headline only)
19/12/20238:17AM Dow JonesNeuren Pharmaceuticals Price Target Raised 33% to A$27.10/Share by Jefferies (headline only)
19/12/20238:17AM Dow JonesNeuren Pharmaceuticals Target Price Raised 19% to A$27.23/Share by Wilsons>NEU.AU (headline only)
Link to release: Phase 2 trial results: significant improvement
Highlights:
- Significant improvement was assessed by both clinicians and caregivers across multiple efficacy measures
- Improvements were consistently seen across clinically important aspects of Phelan-McDermid syndrome, including communication, behaviour, cognition/learning and socialisation
- Clinician and caregiver global efficacy measures showed a level of improvement typically considered clinically meaningful:
- Clinical Global Impression of Improvement (CGI-I) - mean score of 2.4, with 16 out of 18 children showing improvement assessed by clinicians
- Caregiver Overall Impression of Change (CIC) – mean score of 2.7, with 15 out of 18 children showing improvement assessed by caregivers •
- For 10 out of 14 efficacy endpoints, improvement from baseline on overall/total scores was statistically significant (Wilcoxon signed rank test p<0.05)
- NNZ-2591 was safe and well tolerated, with no clinically significant changes in laboratory values or other safety parameters during treatment
From Fintel and Nasdaq
Neuren Pharmaceuticals (ASX:NEU) Price Target Increased by 5.30% to 20.42
The average one-year price target for Neuren Pharmaceuticals (ASX:NEU) has been revised to 20.42 / share. This is an increase of 5.30% from the prior estimate of 19.40 dated October 31, 2023
The price target is an average of many targets provided by analysts. The latest targets range from a low of 17.17 to a high of 23.93 / share. The average price target represents an increase of 37.07% from the latest reported closing price of 14.90 / share
What is the Fund Sentiment?
There are 25 funds or institutions reporting positions in Neuren Pharmaceuticals. This is an increase of 6 owner(s) or 31.58% in the last quarter. Average portfolio weight of all funds dedicated to NEU is 0.13%, a decrease of 4.72%. Total shares owned by institutions increased in the last three months by 8.99% to 4,880K shares
DISC: Small position Held in SM & RL
Acadia just released Q3 results and Daybue sales were $66.9m with Q4 sales of Daybue anticipated to be $80-$87.5m
Very solid on the high side of estimates.
Disc Held
A few quick takeaways from the $NEU SM meeting with Jon just concluded (in these I am purposefully not going over ground covered in previous SM discussions, but have to give a big shout out to @Nnyck777 for great insights which broight me to the party!
- $NEU appear commercially very astute - getting back the 4 indications for NNZ-2591 in doing the $ACAD ROW deal is a master-stroke
- Building a sales, marketing and supply chain capability in orphan drugs is less of a challenge than mainstream pharma or medical devices because of the patient community and small number of specialists who support them
- Competition - I perceive this a lower threat than I have discussed earlier, but I will still be watching the Anavex Phase III result
- Its hard to see $NEU not getting an acquisition offer if NNZ-2591 Phase II Phelan-McDermid read-out is positive. (I hope they have a good defence valuation logged with the Board, as that is the biggest risk to long term holders.)
- NNZ-2591 in the success case is multiples of trofinetide, and $NEU aim to maintain exposure to more of the total value. But success is not assured. However, if it yields a positve impact in humans for P-McD in the PII readout, then that potentially de-risks the other indications given the mouse model findings. (December is a BIG potential catalyst.)
Investment milestones: what I am focused on going forward:
- Attend $ACAD results call in late-Oct/Early Nov (Q3 sales, Q4 outlook)
- Await NNZ-2591 Phase II readout in December
- Monitor for news of regulatory filing for Trofinetide in Europe and/or Japan
- $ACAD annoucement of sale of PRV (and value)
- Monitor Anavex Phase III report towards year end
1. and 2. are both potential material SP re-rate catalysts, and would also drive my holding. A negative result in NNZ-2591 is not a thesis-breaker and, if there is a SP pull-back, could even present a further buying opportunity.
I know I am sitting on a SM valuation of $20; but that is a top-down scaling back of results of a range of scenarios which are actually $12.50-$58.00, which assumes nothing from Fragile-X or NNZ-2591. Rather than settle on a single number, I think it is better at this stage to consider the range. I will update my model post-Q3 $ACAD results.
I've taken another bite of $NEU, and will consider more at milestones 1. and 2. above.
Very happy to have this risk-reward profil in my portolio.
Disc: Held in RL (2.4%) and SM
$NEU reported their 4C this morning;
Their Highlights
DAYBUE™ (trofinetide) launched by partner Acadia in the United States on 17 April 2023 as the first treatment ever approved for Rett syndrome:
• US$40 million milestone payment received by Neuren on first commercial sale
• Highly encouraging early progress, with expected net sales of US$21-23 million in Q2 2023 and US$45-55 million in Q3 2023
• Neuren to receive quarterly royalties on net sales, plus milestone payments of up to US $350 million subject to achievement of annual net sales thresholds, plus one third of the market value of Rare Pediatric Disease Priority Review Voucher awarded to Acadia
Transaction executed to expand trofinetide partnership with Acadia from North America to worldwide:
• US$100 million up-front payment (received by Neuren on 27 July)
• Neuren to receive additional potential milestone payments of up to US$427 million, plus royalties on net sales ex-North America
• Expanded partnership leverages Acadia’s unique knowledge and expertise from the successful development and commercialisation of DAYBUE in the United States
Enrolment completed in Phase 2 clinical trial of NNZ-2591 in Phelan-McDermid syndrome, top-line results expected in December 2023
Commenced Phase 2 trial of NNZ-2591 in Prader-Willi syndrome
A$45 million net cash generated from operating activities in half-year to 30 June
A$87 million cash at 30 June 2023 (A$226 million adjusted to include US$100 million up-front payment received in July)
My Take Aways
I'll keep it brief, as we've covered a lot about $NEU here in recent weeks. This is really all about the key milestones in the 6 months ahead: sales by Adacia in Q3 and (hopefully) aQ4 forecast; sale or use by Acadia of the PRV; the progress on NNZ2591 Phase 2 CT with first potential milestone before year end; and any newsflow of filings for DAYBUE outside USA.
Starting the Q with $39m, with outflows excluding tax of less than $9 and receipts of alomst $60m (driven by deal and first sale milestones), $NEU ends the quarter in a strong case position of $86m and has, in recent days, received a further $100m, to now be sitting somewhere around $186m. With strong sales flowing and further milestones ahead, this should increase over the next year as they build the war chest to pursue further growth.
So, $NEU is where it needs to be to continue to execute its clearly articulated strategy.
Held: RL and SM
In March this year Skyclaris the new Freidrich’s Ataxia drug developed by Reata achieved FDA approval.
The comparison is interesting like Neuren’s Daybue this was the first treatment for the condition. Like Daybue the numbers of potential patients was extremely similar in the US - around 5000. Estimates of 22,000 world wide were suggested.
Pricing is almost exact with Skyclaris costing $370,000 annually vs Daybue’s estimation of $375,000 annually per patient.
Reata’s share price jumped from $31 to $84 per share on the day of FDA approval with estimates of the drug revenue bringing in $1 billion to $1.5 billion by 2030.
As discussed here these revenue multiples are similar if not slightly lower than what Acadia is expecting to bring in based on Daybue alone. Yet the share price today for Acadia sits at US $29 per share and Neuren sits at AUD $13.00.
Why Reata is such an interesting comparative case study is that Biogen has announced today that it will by the company for $7.3 billion. Skyclaris is the only advanced drug in the companies pipeline, with other drugs in phase 1.
There is no doubt that Neuren’s phase 2 studies are pivotal in December. If Reata can command $7.3 billion for 1 drug with estimated revenue similar to Daybue in Rett’s alone. Imagine what Neuren could command for NNZ2591 a drug with 4x the population size across multiple conditions. Daybue will also have likely expanded use cases.
I have no doubt that Neuren is being watched very closely.
As part of continuing to educate myself more about the value of $NEU, which rests largely on the progress of trofinetide ("DAYBUE"), I found the following overall picture of the competitive landscape for the development of the treatments for Rett Syndrome from the website of the International Rett Syndrome Foundation.

Trofinetide took 20 years from discovery to commercial launch, and a decade from reaching the Phase 2 milestone. This is because it is particularly hard to recruit significant numbers in this therapy area. So most of the above candidates appear to be a long way away, even if they succeed (5, 10 years or even longer). Many won't make it at all.
All but one, Anavex2-73, which is being developed by Anavex Life Sciences and is now in a combined Phase 2/3 clinical trial, with a readout in H2-2023. That result is likely to be a (potentially very) significant share price catalyst for $NEU.
Last week, we were trying to understand why the market was taking a less enthusiastic view of $NEU that can be argued as justifable when trofinetide is considered on its own - as @Nnyck777 and @Slideup clearly demonstrated in the "Modelling" forum. Now I think I may have found a plausible explanation. Should a second therapy with an advantageous efficacy profile emerge, the protections of orphan drug status can be revoked, and the competition can then significantly impact the realisable price in the market, even if the front runner has a year or two's headstart. Of course, the target market is relatively small and the competitor also has to generate a return. With two products in the market duopolistic pricing would apply, so the prices wouldn't collapse completely, but they would fall and there would be competition for global share, albeit relatively orderly.
The positive news of the above picture is that additional competitors would appear to be much further away. So even in the success case for Anavex2-73, the opportunity for $NEU could still be attactive. And of course there are the other possible indications and the progress of NNZ-2591, which we have not evaluated.
So any valuation of $NEU has to price in some probability of the emergence of a competitor. I know even less about Anavex 2-73 than the little I know about trofinetide, however, what i can say without fear of contradiction is that shareholders of $NEU, need to be following Anavex Life Sciences, and the readout from the Phase 2/3 trial.
In Feb-22, Anavex reported that Anavex 2-73 met the primary and secondary endpoints in a Phase 3 trial in adults. The key for Rett Syndrome is to prove efficacy in children, because a large part of the value lies in managing the symptons and condition so as to allow a more normal developmental pathway for children with the condition (as I understand it). Trofindetide has proven this, and it has achieve a broad FDA-approve label with no requirements for ongoing monitoring. That is in itself quite unusal in treatments for rare diseases and creates a high bare for Anavex 2-73.
I will likely hold off on any decision to increase my initial $NEU position until the competing trial reports later this year, unless there is positive newsflow on NNZ-2591.
See the slide below from Anavex Life Sciences corporate presentation, which is relevant.
I wonder if this is a case of: when the market is telling you something, you have to keep digging?

Disc: $NEU held in RL (1.9%) and SM
Looking at future revenue here established in previous tables posted.
3 years $440 million revenue less expenses say earnings $300 million/ 126 million shares on issue x P/E of 50 (reasonable for growth biotech on asx) = $119.00 a share
with more conservative P/E of 25 still $60 per share.
I will post the lower end to be conservative.
Neuren Numbers
I recommend the following video to understand the significance of the new deal with Acadia:
https://www.youtube.com/watch?v=_bMto7lvoEE
This slide presentation matches the video:
Neuren Rett Syndrome Estimates in the US alone
5000 Currently identified people
10,000 Potential

Rett Estimates for the ROW (rest of the world)
Neuren has provided very rough estimates of potential Rett numbers in Europe, Japan, China Urban and Other Asia.
To minimize complexity I am going to just concentrate on Europe and Japan.
4000 Currently identified people Europe
13,000 Potential in Europe
1000 Currently identified people Japan
3000 Potential in Japan

Pricing Rett ROW for Models
I am keeping this very basic. Google tells me based on a 2021 study by Rand corporation that prescription prices of drugs in the US are 2.4 x higher than in Japan and other European Countries such as Germany.
So I estimate $US150,000 per annum per child in Europe and Japan in my model (as opposed to $375K per annum in US).
Also milestone payments accrue with the addition of ROW numbers, so milestones payments will be accrued faster. Milestones however are only paid once. The tiered royalties are exactly the same as the US percentages for Daybue.
This will stay the same independent of whether Daybue is used for Rett or other indications such as Fragile X.
Fragile X Estimates in the US alone (Figures sourced from Neuren)
30,000 potential people in the US with Fragile X
It is still unclear whether Daybue will be developed for Fragile X or whether Acadia will develop NNZ2591 for this condition instead.
As this is unknown I have left it out of the model
Fragile X will need a Phase 3 study with Daybue so 1 year plus 6 months fast track FDA approval. So conservatively no revenue from Fragile X until 2025.
Fragile X Estimates for the ROW (figures sourced from Neuren)
38,000 potential people in Europe
117,0000 potential in Asia
Time Horizon for models
Daybue patent exclusivity to 2040
My model assumes only Rett revenue until more details emerge around fragile X.
Important note on milestone payments
Jon Pilcher has confirmed that milestone payments are one off, once paid they are no longer factored into Neuren’s future revenue.
Neuren’s Current Position Factoring new announcement.
The first major rejig of models came on FDA approval when analysts estimated that Daybue would cost $180K per annum per child with Rett.
The surprise to the upside was huge with cost estimate per child based on average weight of $357K per annum per child.
Remember too that all these figures are in US dollars which of huge benefit to Neuren currently. I am assuming an exchange rate of 0.68c.

*Converted to AUD above in Table
So cash on hand for Neuren as of July 2023 is AUD $240 million
The PRV has not been factored in. If Acadia use or sell this soon this Cash on Hand balance increases to AUD $297 million.
My revenue model estimates prior to this new Acadia deal.
I estimated Acadia was on track to receive $US 76,500,000 or $AUD 112,500,000
However this estimate already appears to have been exceeded according to Acadia with Q2 23 million Q3 55 million reported revenue estimates. Say conservatively Q4 sees 70 million. That suggests that they were able to rollout Daybue to approximately 400 people in the first 3 qrts since release. A first year estimate of 500 patients seems very possible.

*All figure is US dollars
**0.68c currency conversion (I will guess at this but keep it consistent, although this will account for error in the model)
Remember Milestone Payments are triggered every calendar year. So 2024 is unlikely to trigger this.
However my revenue estimates for 2024 for Neuren are $27 million (tiered royalties) + $146 million for the new ROW payment deal + $50 million with likely sale of PRV.
I don’t believe that any ROW numbers will be rolled out in the calendar year of 2023 so this has been excluded from estimated revenues. I believe these will be involved in calendar year 2024.
FY 2024 Total Revenue = AUD $223 million which is well ahead of estimates of $120 million.
Priority Revue Voucher (PRV) Notes
Acadia still retains the priority review voucher.
Priority review vouchers are only valid for one drug molecule.
Total resale value is approx. US$100 million.
If Acadia uses this voucher themselves then will still need to pay Neuren 1/3 of the value.
If Acadia sells this voucher they will still need to pay Neuren 1/3 of the value of the voucher.
Neuren will receive US$33 million or approximately $50 million AUD.
Unsure of the timing.
With approval of NNZ2591 there will be a second PRV distributed by the FDA. Jon has mentioned that this is likely to go to Neuren as they are more advanced in their pipeline – up to phase 2 trials already for this molecule. So they perhaps will receive the full US$100million on approval.
However if Acadia does somehow receive approval first for NNZ2591, say for Fragile X, Neuren will still receive 1/3 of the value of their voucher.
The importance of the New ACADIA NEUREN DEAL just released.
Neuren and Acadia’s New Deal Payment Structure

1. More cash in the bank immediately contributing to FY 24 revenue
2. Upfront payments for commercial sales likely to be triggered in both Europe and Japan in calendar year 2024 as Daybue data is so comprehensive and well understood by ACADIA and there is an urgent unmet need for this condition.
3. Fragile X re-immerging as a new revenue stream for Acadia and hence Neuren
4. The very significant removal of restriction of NNZ2591 being restricted for development by Neuren in Rett and Fragile X
5. Future payments for NNZ2591 for Rett and Fragile X from Acadia to Neuren, secured at a much higher % of royalties as are ROW for Daybue
6. Neuren retains the full world rights for NNZ2591 for all other conditions including:
a. Phelan McDermid (US 22,000 EU 28,0000, ASIA 81,000)
b. Angelman Syndrome (US 14,000 EU 18,000 ASIA 52,000)
c. Pitt Hopkins (US 7000 EU 9000 ASIA 25,000)
d. Prader Willi (US 13,000 EU 18,000 ASIA 47,000)
Restrictions have been lifted on what can be developed by Neuren.



NNZ2591- Due for Phase II readouts in Dec 2023.
However it is an open label study so Acadia and Neuren would be well aware of the first signs of results during negotiations for this drug.

New Models To Include Rett for US, EU and Japan



Basic model for all sales and milestones and upfront payments by year

This does not take into account Fragile X or NNZ2591 development.
Like all models I am plucking numbers of potential takers of the drug assuming that most will stay on it. It is a really hard thing to model. So I presume this is a very rough guideline only.
Would be interested in others perspectives.
However it might help with rough estimates of uptake in different territories.
Thanks
Nnyck
NEU a baby giant
I really enjoyed the baby giants podcast interview with Pro Medicus founder Sam Hupert this week. I couldn’t help but find similarities to another Australian company that I think, like Pro Medicus, will grow to be an industry giant.
A bold claim I know but let me share some thoughts on the company, its future revenue and potential.
While listening to the podcast a few things dawned on me. While the two companies are very different medical software versus drug development, there are some key similarities. It is by studying the beginnings of other baby giants that we can learn how to spot the birth of a new giant.
Like Pro Medicus, Neuren Pharmaceuticals has become fully self -funded with the success of its lead drug. Like Pro Medicus there is no debt. And now oodles of cash on hand thanks to a successful FDA approved drug. Neuren Pharmaceuticals has an excellent fiscally responsible and smart leadership team and board. CEO Jon Pilcher, like Sam, is strongly aligned with share-holders and heavily invested in the company himself.
Neuren the ‘overnight success’ like Pro Medicus has actually been around a long time and was founded in 2001. It has been a long dull road for share holders until recently. I met a few of these share-holders at a Strawman meeting in Brisbane and it was Trevor and these guys who first peaked my interest in the company.

As with a lot of biotech companies you can put your money in and feel like you are watching paint dry, as very little happens for a long……. time. Around the time I caught up with these investors there was a lot of insider buying creating interest, plus phase 3 clinical trial results for Trofinetide were due that year. Share-holders were getting pretty excited. As it turns out, rightly so.
FDA approval is a big deal for an Australian biotech company. However other Australian companies have done this before and as we will see not all of these companies turn out to be industry giants.
I think Neuren’s model, their ‘sweet spot’ is what makes them unique and a potential giant. Let me explain; Neuren Pharmaceuticals has 2 lead drug assets and the beauty of these drugs is that they have been shown to improve the distressing symptoms seen across multiple neurodevelopmental disorders, like hand wringing. Trofinetide now known as Daybue and NNZ-2591 are different molecules but both work by repairing impaired connections and signalling between brain cells.
The implication is that both drugs can stop or reduce neurological symptoms across a multitude of conditions, while a patient is on them. Deficient signalling between cells can cause behavioral and cognitive problems, deficient motor skills and breathing and cardiovascular problems.
Neuren’s drugs are a golden opportunity as they treat rare (not super rare) disorders that have no current treatments available. Hence, they are more likely to get approval and once they do, they can yield much higher drug prices as they are considered a cost effective investment by Medicaid and US insurers. Children end up spending a lot less time in hospital from their symptoms which is viewed as a major cost saving.
Daybue is now approved for children 2 and up for the treatment of Rett Syndrome. However this drugs success is unlikely to stop there as It also has the potential to treat the symptoms of other developmental disorders such as Fragile X. It should be noted that Daybue is also patented as a treatment for autism in Israel.
We will look at the expected revenues for Daybue for Rett Syndrome further down. What I want to emphasize is that this drug is patent protected until 2039. So there is plenty of time to generate revenue and to look at other potential applications in the neurodevelopmental world.
What is more exciting is the second drug NNZ-2591 touted by Jon as the ‘diamond’ of the two drugs. This is already in phase 2 clinical trials for 4 childhood neurodevelopmental illnesses. Read outs for these studies are due as early as July this year. This drug has been described by one rather excited share-holder as ‘aspirin’ for the brain. While a little exuberant in their description, I don’t think that their excitement is unfounded.
While 4 childhood conditions are being targeted for now, there is potential that numerous other conditions such as autism could be treated.
Having an FDA approved drug on the ASX is a rare feat with only a few Australian companies achieving this coveted goal. Obviously, FDA approval opens the door to a much larger market in the US and much higher drug pricing. Companies that have walked before Neuren and lit the way include: Tellix Pharmaceuticals (Illucix 2021), Pharmaxis (Bronchitol 2020), Clinuvel (Scenesse 2019), Hatchtech (XeglyzeTM 2020), Medicines Development for Global Health’s (Moxidectin 2018).
A quick look at these predecessors shows what can happen to the share price of biotech companies when FDA approval is granted.
Illucix, Telix Pharmaceuticals drug offering to treat prostate cancer was granted FDA approval in December 2021. There was a big increase in share price pre-approval followed by steady gains to highs in January 2022.

Pharmaxis did not see such a share price increase when Bronchitol received FDA approval for its Cystic Fibrosis inhaler. In fact it only increased from 0.09 to 0.10 cents per share and largely flat lined from there. Pharmaxis partnered with Chiesi Farmaceuticals in the US to complete phase 3 trials and received milestone payments from Chiesi on FDA approval. It continued to receive high teen % royalties on US sales of Bronchitol. The company acknowledged a huge impact of COVID 19 on their distribution and sales capabilities.

World-wide sales were over $5 million in 2022 but growth had largely flat-lined and this figure was similar to total revenue from 2021. Share-holders were not happy about this.

Gary Phillips the CEO delivered a share-holder address in 2022 that detailed the challenges of selling Bronchitol to the US market. Pre – covid cystic fibrosis patients attended clinics regularly at least 4 times a year and post covid only 2 times a year. Hence Pharmaxis had less opportunity to deliver the respiratory test required before Bronchitol prescription.
Pharmaxis (PXS) market cap is currently around $32 million. PXS revenue from sale of goods (full year to June 30, 2022) was $7.42 million. Cash on hand is close to $20 million with the recent sale of the Orbital device. However, Pharmaxis made a loss of $1.93 million in FY22 but this was an improvement on the previous financial years loss of $3 million.
On a side note PXS Parkinson’s drug to treat sleep disorders in early stage disease looks promising. It is currently in Phase II studies. Their drug to reduce skin scarring is due for data read out in mid 2023.
PXS although not a shining example of share price accretion post FDA approval or conversion to an industry giant, is a company worth further investigation. It appears to be self-funding through sales and grants with decent cash on hand and a very interesting and diverse product pipeline. It certainly seems to have a very low market capitalisation. The CEO believes that the share holders are punishing the company for disappointing Bronchitol sales.
Clinuvel a company I know many Strawpeople have followed has been pretty successful post FDA approval for Scenesse. Since approval in June 2018 the share price has doubled. Not jaw dropping gains but 100% returns are certainly nothing to sneeze at.

Scenesse treats Erythropoietic Protoporphyria. This is an incredibly rare disease affecting approximately 1 in 75,000 people. In Europe Scenesse was priced at $109,730.00 per patient annually.

(from Clinuvel Fy 2022 announcement)

(from Clinuvel Fy 2022 announcement)
While Profit is certainly increasing Opex is also increasing. Clinuvel since FDA approval is certainly self -funding and paying a dividend. It is also re-investing in research and development. Total revenue generation was $66 million in Fy2022 with roughly $20 million after tax profit. Certainly an impressive growth for an asx biotech. However Clinuvels figures will likely be dwarfed by Neuren’s revenue and impending profit from Daybue.
XeglyzeTM the Australian nit treatment had a very different road to development. Hatchtech is not listed on the ASX. The drug took 20 years to develop and $40 million in investment money to get to FDA approval in the US. Uniseed was the initial investor and Dr Reddy’s Laboratories (NYSE: RDY) became the US commercial developer.
This private equity deal was touted as a huge success for an Australian biotech developer. Hatchtech received an up-front payment of US$10 million and up to US$50 million in pre-commercialisation payments. There were additional milestone payments on commercial sales of up to US$137 million. Dr. Reddy’s was the official distributor in multiple countries including USA, Australia and Canada. However, Hatchtech did retain the rights to distribute XeglyzeTM across several other countries. While impressive this company did not become an industry giant.
Moxidectin was another FDA approved drug to treat river blindness. It was developed by a not for profit group Medicine Development for Global Health 2018. While an impressive development group it is also not a publicly listed company.
From their website:
“Our expertise is in the product development process, which we apply to advancing medicines and vaccines that address important unmet medical needs, predominantly in low- and middle-income countries. We work with global colleagues, collaborators and contributors at all stages of the process of investigating and implementing new and improved medicines for the treatment of neglected diseases. “

Clinuvel certainly appears to be the most impressive comparison. CUV is generating growing revenue and it is profitable and dividend paying. Neuren will be profitable and similarly reinvest into clinical trials and Jon Pilcher stated that it is not looking to pay dividends but to grow their product pipeline (listed below).

(table above from Neuren website)
Neuren’s Daybue deal was back-ended and is now looking like a very clever move from Jon and the team.

(table above from Neuren website)
Neuren as of December 2022 had a cash balance was $40 million (includes US $10 million for NDA acceptance). Neuren is about to receive another US$40 million milestone payment on first sale likely in April (AUS $61 million Australian). Roughly $100 million in the bank. At some point they will also receive 1/3rd of the value of the sale of the Priority Review Voucher. This will be an additional amount directly to Neuren’s bottom line of AUS$ 50 million). This will be a total of AUS$150 million before recurring percentage royalties are factored in.
Acadia is in charge of commercialisation, marketing and distribution. This Opex will be carried by Acadia alone and Neuren will not have any expenses for Daybue incurred. Neuren will incur usual operational costs plus additional costs for funding Phase 2 clinical trials.
The way the deal was reached meant that Acadia would have to pay Neuren one off sums if sales exceeded a certain amount in a calendar year.
Minimum Sales Scenario
US $250 million will mean US$25 million (10%) to Neuren and US$50million in milestone payments.
This will total a minimum of AUS$115 million in % sales and milestone payments.
Moderate Sales Scenario
US$500 million in sales US$55 million in % sales and milestone payments of US$100 million triggered.
This will total a minimum of AUS $238 million in %sales and milestone payments
Best case Scenario
US$1bln in % sales US$128million and US$350 million in milestone payments. This scenario will require roughly 3000 Rett patients (3000 x 375K) to be using Daybue (estimated 10,000 patients with Rett in the US.
This will total a minimum of AUS $196 million in %sales and AUS$538 million in one off payments.
Certainly a more attractive revenue and profit potential when compared to Clinuvel.
If a new treatment indication for Daybue is found (say Fragile X) these sales will also contribute to these sales targets increasing the likelihood that milestone payments will be met.
The surprise this week was that pricing models for Neuren’s success was based around US drug pricing of $180k per patient per annum. The result surprised to the upside which left analysts needing to revise their modelling. Acadia has achieved pricing for Daybue of US$375K per annum. This will be covered by Medicaid and Insurers with only 3% being paid directly by families.
Neuren looks like it is going to be a hugely profitable company off the back of their deal with Acadia.
This baby giant has a plug and play model for 4 other conditions and if successful will likely achieve much higher returns due to the higher number of children suffering from these other conditions.
Neuren is now also in charge of a rest of the world deal. Jon and team are planning to sell rights for Daybue to be distributed in other countries outside of North America. Jon is currently talking to interested pharmaceutical companies. An announcement about this new revenue stream could come any day.
There are limited examples for comparison on the ASX and Neuren certainly looks like it will be entering its own league. Like Pro Medicus there is a wide Moat as the areas they are treating have orphan drug status and no current treatments are available. This makes Neuren a unique and potentially very lucrative profit generating biotech company - dare I say it......a likely industry giant.
Cheers
Nnyck
10-March-2022: (USA Time): U.S. FDA approves Acadia's genetic Rett syndrome drug | Reuters
Plain Text Link: https://www.reuters.com/business/healthcare-pharmaceuticals/us-fda-approves-acadias-genetic-rett-syndrome-drug-2023-03-11/
Excerpt:
March 10 (Reuters) - The U.S. Food and Drug Administration [FDA] approved Acadia Pharmaceuticals Inc's (ACAD.O) drug for the treatment of Rett syndrome, a genetic brain disorder, the company said on Friday, making it the first approved drug for the condition.
The U.S. health regulator's decision allows use of the trofinetide, to be sold under the brand name Daybue, in adult and pediatric patients two years of age and older and comes with a warning of diarrhea and weight loss.
The approval comes months after the FDA declined to approve expanded use of Acadia's drug Nuplazid to treat psychosis related to Alzheimer's disease. Analysts have said approval of Daybue would help drive growth for the company in the near term.
"We have put a lot of planning into potential commercialization of trofinetide, including resources for patients to access the drug," said Acadia senior executive Kathie Bishop ahead of the approval.
Acadia said it plans to make the drug available to patients by the end of April. It did not disclose details of the price.
Before the approval, David Hoang SMBC Nikko Securities analyst estimated a list price at launch of $450,000 annually. He forecast peak U.S. trofinetide sales of $487.2 million by 2035. RBC Capital Markets analyst Gregory Renza, also writing before the approval, predicted peak U.S. sales to exceed $500 million by 2032 and an average annual launch price of about $425,000.
Acadia forecasts sales of Nuplazid - its only drug on the market - of between $520 and $550 million this year, above analysts' median expectations of $532.8 million, according to Refinitiv data. With the drugmaker facing a loss of exclusivity for Nuplazid in 2028, investors have pinned their hopes on a successful trofinetide launch.
After the FDA declined to approve the expanded use of Nuplazid, Acadia said it would not pursue that indication for Nuplazid further. The drugmaker plans to focus its resources on late-stage development of Nuplazid to treat symptoms of schizophrenia and early-stage development of another candidate, ACP-204, for Alzheimer's-related psychosis.
Rett's syndrome is a rare neurodevelopmental condition that occurs primarily in girls. According to government estimates, it affects fewer than 50,000 people in the U.S.
Shares of the California-based company closed 0.68% lower on Friday.
Acadia's drug acts as an artificial form of the insulin-like growth factor IGF-1 and helps reduces inflammation in the nervous tissue as well as aid in the transmission of nerve impulses.
Acadia's application for marketing approval was based on data from a late-stage study in which treatment with the drug showed improvement in core symptoms of the disease compared to a placebo. Improvement of symptoms was measured according to the assessment scales Rett Syndrome Behaviour Questionnaire and the Clinical Global Impression of Improvement.
The company had licensed the drug for $10 million up front payment from Australian drugmaker Neuron Pharmaceuticals (NEU.AX) for development and sale in North America in 2018.
--- end of excerpt ---
Source: Reuters.com: Reporting by Bhanvi Satija, Nandhini Srinivasan and Anirudh Saligrama in Bengaluru; Editing by Shailesh Kuber and William Mallard


Watch for an announcement from NEU on Monday or Tuesday morning. Share price expected to move, by how much I do not know. Not holding.
It's a public holiday on Monday in SA (Adelaide Cup Day), the ACT (Canberra Day), Victoria (Labour Day) and Tasmania (Eight Hours Day), but importantly not in WA, Qld, the NT or NSW (where the ASX is HQ'd). It is however a public holiday in NZ (Taranaki Anniversary Day) and the two head office addresses I have found for Neuren are (1) in Auckland, New Zealand (according to the ASX and Commsec) and in Camberwell, Victoria (according to Neuren's own website), so that's why I said Monday or Tuesday morning, seeing as it's a public holiday in NZ and Victoria on Monday. Hopefully, somebody can work-from-home or drop into the office anyway to email a short letter through to the ASX announcements platform.
Regardless of whether the announcement comes through on Monday or Tuesday, expect some SP action on Monday.
