Consensus community valuation
Straws are discrete research notes that relate to a particular aspect of the company. Grouped under #hashtags, they are ranked by votes.
A good Straw offers a clear and concise perspective on the company and its prospects.
Please visit the forums tab for general discussion.
21 October 2025
$0.18 ($0.08 - $0.50)
Method 1
Updated valuation based on reductions to GTN and low case TRx trajectory indicating reducd peak revenue (Scenarios of AUD90 m - $AUD200 m in FY28).
Other assumptions: single formulation; dispensing via dermatologists only; US only; no indications beyond PAHH; no telemarketing contribution; no new products.
Disclaimer: Valuation is highly uncertain as based on projections of only 9 months of sales data from a single product company. Do not use as a basis for your investment decision. (Always true, but especially today!)
Method 2
$0.16 market price realised on 20 October 2025.
24 July 2025
$0.35 ($0.22 - $0.90)
Full valuation update following new model based on 8th July Webinar.
See Straw for full description of valuation.
Disclaimer: Valuation is highly uncertain as based on projections of only 6 months of sales data from a single product company. Do not use as a basis for your investment decision. (Always true, but especially today!)
15 April 2025
$1.10 ($0.80 - $1.40)
I'm not doing a model update as we are still in the world of pretty wide uncertainty, and so I've just done a little rounding of my numbers to get rid of the spurious accuracy.
However, the update is to prevent this valuation getting stale.
Based on today's Webinar and the plan to accelerate sales and marketing scale-up, as well as the sales trajectory in the early weeks, I am feeling very comfortable with the lower end of the valuation range, per my alternative analysis last week.
So this is no longer a Bull Case (as indicated below), as the Bear scenario is essentially de-risked. The product has translated to the US. Yay.
The next key valuation catalyst will be the FY25 report and how the sales trajectory has responded to increased investment, That's said, I imagine earlier market updates are likely.
21 May 2024 - Bull Case (SOFDRA now approved)
Updating the valuation based on the 14th May assumptions, now with Sofdra approved, as follows.
SOI now 1,575+233, and allowing for further dilution due to share options to 2029, giving assumed 2029 SOI=2,000m
No change to 2029 EPS, debt free, and tax rate
Eliminate FDA failure cases and resubmission scenarios
Retain 12% discount rate to reflect market uptake risk.
2029 EPS now $0.055
Gives 2029 values for P/Es 25 & 45 of $1.36 - $2.45
Discount back at 12% to 2024 gives $0.77 - $1.39
Add back in value of $70m cash of capital raised = $0.035/share
Valuation range: $0.81 - $1.43
Central Case $1.12
Which kinda explains the limited SP movement today, versus what might have been expected, given that my pre-approval, undiliuted SP was $1.13.
----------------------------------------------
14 May 2024 - Bull Case
See today's Straw for full justification.
On the basis that $BOT achieve an EBIT in 2029 of US$104m, carrying no debt, and applying tax at 30% and USD:AUD 0.67 give 2029 NPAT of A$109m.
With 1,575m SOI, although $BOT will be highly cash generative quite soon, I'll allow some dilution due to share based compensation, so assume SOI of 1,800 in 2029.
That gives a 2029 EPS of $0.061.
I'll deal with the uncertainty via the P/E ratio, ranging from 25 to 45 - probably very conservative for a high growth pharma company.
I'll add a risk premium to the WACC, and discount at 12%.
My unrisked valuation range is: $0.88 to $0.1.56 (but including a margin of safety in the risk premium)
So, now I am going to apply my 90% CoS, and assume that in the 10% failure case
- There is a net 5% chance that there is a subsequent approval on whatever the residual issues are, and that the profile gets pushed out by another year, leading to a further discount and a further dilution of 10%.
- There is a net 5% chance that the drug is withdrawn amd the value of the business is $0.06 of the development portfolio.
Boiling all this up together, and I get a risked valuation of: $1.13
Just wanted to update my previous model based on the most recent 4C data to confirm what at first glance looked ugly.
Despite the confusing change in metrics I have come up with the following, could be right or wrong..
From the 4C, There were 20417 total units shipped in Q1,
** only thing that may skew this is August potentially was low due to the holidays or whatever goes on over there, the pharma data on HC seemed to support this from the bloomberg terminal or where ever it comes from, this is possibly what got it pumped down to 10c recently.
** In the below table:
-- Yellow, total units shipped,
-- July units = historical June units (which they gave previously of 5474 or thereabouts) + X
-- August = July + X
-- Sept = Aug + X
Goal seek X for when Q1 Total = 20417: 666 net additions per month.
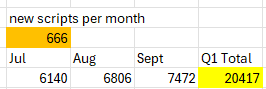
Previous posts I was thinking that 1000 / month would be a good base with increased sales force / prescriber productivity etc but missed this by 40% and also the GTN is in the shocker.
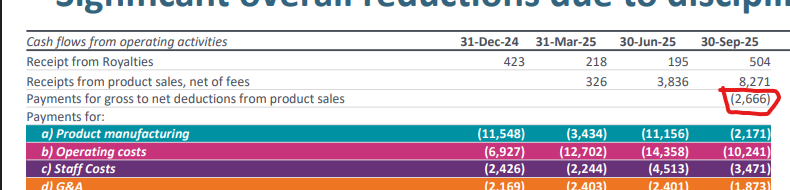
Also this little expense line has been fleshed out of somewhere, not sure what it fell under previously but makes the net revenue $5.6m?
anyway, based on the old model with updated numbers...
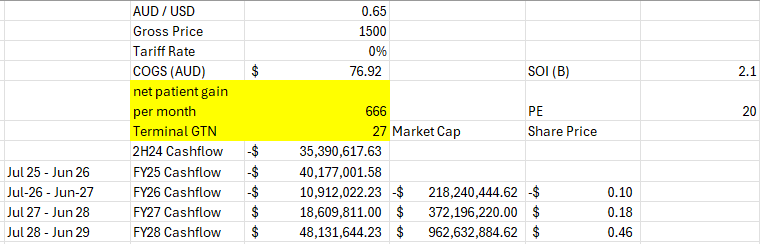
COGS I possibly have low at 77 / unit AUD but who knows with what they are reporting.
no COGS until Jan 2026.
-40m loss this FY probably more if winter and high deductible season hit hard.
-11m loss the following year
maybe an 18m profit the year after before tax.
I guess there is a chance the new reps get some growth firing but that net patient gain I have deduced from the numbers provided is ugly to me as is the GTN. my theory about the GTN and PA units must have been incorrect.
Hard to figure it out when they have taken away the script breakdown between free, reimbursed, PA etc.
again that was the only red flag I needed this morning to unload what I had left, they changed the metrics because if it actually is 666 per month average net patient gain of the quarter it stinks more than the tales of $400-450 USD Net / unit.
As a side note, suppose August was a shitter of a month due to holidays etc
If I set August in the previous table to be 60% of the scrips added in July and then goal seek for scripts / month, brings the average up to 765 but with a pretty average August.
Still makes for ugly reading considering where it was hoped to be going.
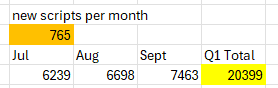
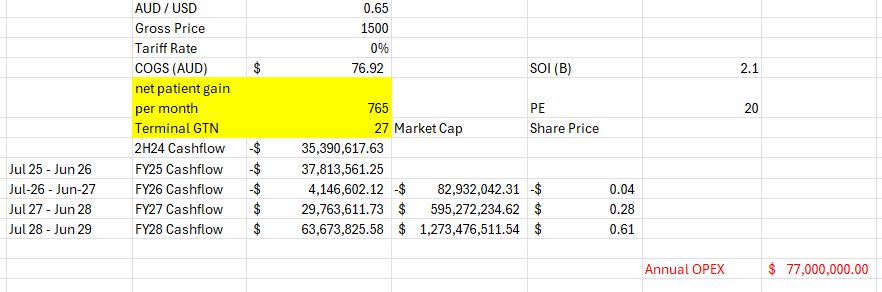
hope it makes sense, can't see too many positives after having looked a bit deeper.
Maybe my OPEX is a bit high, but the COGS is murky at best to me
Maybe my GTN at 27% is a bit low, but its really flattening out month by month
As always any comments welcome hope this may be of use to anyone still holding and on the fence, to me there are much better options out there than to wait and see if they can do it because the performance so far has been underwhelming.
Botanix Webinar – Monday 20 October:
Registration Information Philadelphia PA and Phoenix AZ 16 October 2025:
Commercial dermatology company, Botanix Pharmaceuticals Limited (ABN 70 009 109 755) (ASX:BOT, “Botanix” or “the Company”), announces that it will host a webinar to provide a comprehensive update on the Company’s Quarterly Activity Report and 4C Quarterly Cash Flow Report.
Executive Chairman Vince Ippolito, Chief Executive Officer Dr Howie McKibbon and US Chief Financial Officer Chris Lesovitz will include an update on the launch momentum for Sofdra® (sofpironium) topical gel, 12.45%.
The webinar will be held on Monday, 20 October 2025, at 11.00 am AEDT (Sydney/Melbourne)/8.00 am AWST (Perth).
Interested participants must register before the webinar using the link below. Dialin details will be sent in return.
Date: 20 October 2025 Time: 11.00 am AEDT (Sydney/Melbourne), 8.00 am AWST (Perth) To register: https://us06web.zoom.us/webinar/register/WN_-oGtp6viSHG5NwrKmitAsw Dial in details: Will be sent to you directly upon registration
While several of us in the community are still traumatised by the June "Nightmare on ..." it is worth bracing ourselves for what I expect to be a $BOT Trading Update next week, ahead of the 4C towards the end of the month.
@jcmleng covered the August Canaccord Genuity Conference, and I thought as part of my own preparation, I'd share some extracts from the Canaccord Genuity Analyst Report following that conference (published 1 September 2025, but I've only read it today).
While the presentation gave no new disclosures, which was also the case at the more recent Wainwright Investment Conference (in early September), there was a "fireside chat" format, and so some insights were gleaned from that, which I've highlighted in bold below.
TLDR: I am reasonably aligned with CG. Their valuation of $0.27 compares with mine of $0.35, and I believe the depressed SP at the moment reflects a loss of trust, so that void left by shareholders who fled with their "night terrors" was not been replaced with new believers. If the next update is half decent on scripts, GTN, and revenue, that could be a significant step towards rehabilitation.
Extracts from Canaccord Genity Report (1/9/25)
Summary
"We maintain our BUY rating and $0.27 PT on Botanix Pharmaceuticals. BOT remains in a holding pattern as investors wait to re-establish trust with the expectations around Sofdra traction, particularly as it relates to script volume and gross-to-net yield improvements. We would caution investors not to place too much focus on single data points, however. Our fireside chat with management at Canaccord Genuity's 45th Annual Boston Growth Conference suggested to us that the 30 sales reps now in field are continuing to convert patients at the expected rate. We have therefore maintained our script volume growth and assess the ability for Botanix to meet these numbers as reasonable. There are calculable reasons as to why the gross-to-net yield can sit at ~25-30% within a ~18-month time frame; we have moderated this over FY26e and FY27e to 26-32% (from 29-33%) to reflect this. We view revenue expectations being met through either volume growth or gross-to-net improvement as alternate commercial strategies, rather than preferring either one - with the caveat that we expect profitability to rema intact for 2Q27e. We, and the market, keenly await a trading update in October."
"FY25 summary. Revenue: Total revenue of A$5.8m was largely pre-reported, noting ~A $5.0m directly relates to sales of Sofdra in the US (the remaining attributed to royalties from Ecclock sales in Japan). Sofdra sales reflected ~16,689 total prescriptions (TRx) sold since Jan-25. At a gross price of ~A$1,500 per script (per month), gross revenue sits at A$25.2m, reflecting ~20% average gross-to-net yield across the period. OpEx and earnings: Total OpEx of A$94.1m was 10% ahead of forecasts (CGe: A$85.6m), noting cash OpEx (excl. SBPs) of A$73.5m was only ~4.9% ahead of forecasts (CGe: A$70.8m). Loss from operations therefore sat at A$86.4m (CGe: A$83.4m, -3.5%). Cashflow: operating cash outflow of A$78.6m was driven by a large WC outflow for inventory build (~A$27m) as well as inflows related to R&D tax incentive (A$1.5m) and interest income (A$1.9m) sitting ~$4m ahead of forecasts (CGe: A$82.6m outflow). As reported in the 4C, Botanix closed FY25 with $65.0m in cash, having raised $40m in equity in April-25 and established a US$30m (A$48m) debt facility with Kreos Capital, of which A$31m was drawn down."
"Forecasts and outlook. Our main model adjustments include: a) moderating GTN yield in FY26e from 29% to 26% (A$6m topline), b) adjusting expenses, accounting for a larger SBP expense, c) inventory (noting no inventory build is expected in 1H26e), d) removal of additional debt drawdown. We see the next two quarters as paramount to Botanix reestablishing trust with the market. We expect Botanix to provide a 1Q trading update in Oct-25. For context, we forecast 1H26e net revenue of A$23.2m. The A$23.2m is predicated on two factors: 1) on the basis that June-July growth is the same as May-June growth (~21%); we forecast ~14% MoM growth in script volumes is required to reach our 1H26e number which needs to be coupled with... 2) an improvement in gross-to-net yield. As a reminder, as of June, GTN yield sat at 23%; we need to see GTN head ≥26% (remember 2H of a CY is a stronger GTN period). "

"Valuation. Our diluted 12-month price target of $0.27 is informed by our DCF model (WACC: 10.3%, Tg: 2.5%) and cross-checked against ASX-listed and global comps (median FY+1 EV/Rev: 3.2x), as well as dermatology deal values (median EV/Rev multiple: 3.4x), which sits broadly in line (4.0x) based on FY27e CGe net revenue: A$140m. More importantly, across the forecast period (FY26-FY28e), we believe Botanix has the capacity to build into a peer comparable EV/EBITDA multiple of 8.0-11.0x, with our PT in line with FY28e EV/EBITDA at 7.5x."
My Assessment
Who knows what 1Q revenue will look like, as multiple factors are at play:
1) seasonality (+ or -)
2) evolution of GTN (+)
3) maturing market penetration (-) and
4) expanding sales force and territories (+)
5) increasing prescriber experience in prescribing ... initial cohort entering their second 6-month period. (likely + but could be -)
Revenue is the key unknown, because costs are controllable and management have demonstrated that they know they have to show an improved control of expenses.
I think the CG numbers above are a good reference to check 1Q against. They could be a little bullish, because of the delay in getting new reps up to speed, but as I've shown above that is only one of several factors.
In my assessment, there is a significant margin of safety between today's SP and any reasonable valuation on fundamentals. The discount is really a management credibility one, and as CG state, $BOT will need two solid quarters of execution to start to repair that.
When we get the next management briefing, I will be very interested to hear about the prescribing behaviour in the more established accounts. How that trends will be an important indicator of where we end up in terms of revenue plateau.
At the start of the year, I followed the Chairman by selling 25% of my RL holding at $0.465 (and sold some in SM too). At the time, I feared I was being a wally, given my valuation at the time. But I was unnerved by Vince's sale. It turns out that was a good decision (for Vince and me!)
But in recent weeks, when the SP hit $0.125, I bought those shares back in RL (and also added again in SM) because things would have to go really badly for the business to have that value. Unfortunately, in that case I was on my own with the Directors and Insiders not sharing my enthusiasm.
Management have been very tight-lipped during the last Q. Maybe they were rightly beaten up for all the loose talk early in the year about revenue expectations for FY26, and perhaps the Board resolved "Shut the f*** up and let the results do the talking."
Well, not long until we see what the revenue trajectory is looking like.
(I have to remind myself that I'm sweating more not because I need the product, but because its just warming up in QLD as we head towards the Summer.)
Disc: Held in RL and SM
Not huge news but should bring in a little royalty revenue, when its up and running....
Approval for Marketing Authorization for ECCLOCK gel 5% in Korea
for Treatment of Primary Axillary Hyperhidrosis
Kaken Pharmaceutical Co., Ltd. (“Kaken”, head office: Bunkyo-ku, Tokyo; President and
Representative Director, Hiroyuki Horiuchi) announced that Dong-Wha Pharm. Co., Ltd.
(“Dong Wha”, head office: Seoul; Co-CEOs, Jun Ha Yoo and In Ho Yoon) has obtained
approval from MFDS: Ministry of Food and Drug Safety for the marketing authorization in
Korea of ECCLOCK gel 5%, a topical formulation drug for primary axillary hyperhidrosis
(generic name: sofpironium bromide; product name in Japan: ECCLOCKⓇ).
In June 2023, Kaken and Dong Wha entered into an agreement under which Kaken granted
Dong Wha the exclusive right for the development and commercialization of the product in
Korea based on its rights to sub-license in certain Asian countries granted by Botanix SB, Inc.
(head office: Pennsylvania, USA), and Dong Wha had applied for marketing authorization for
Sofpironium Bromide. Dong Wha plans to launch the product at the earliest possible timing.
About Dong Wha
Dong Wha is a Korean pharmaceutical company, listed on the Korean Stock Exchange
(000020.KS), with strong capabilities in research and development, manufacturing and
marketing and has been providing superior pharmaceutical products in Korea since its
foundation in 1897. For more information, please visit https://www.dong-wha.co.kr/.
Amidst all the excitement last week, I just realised that BOT dropped its FY25 Appendix 4E and Annual Report at about 5.20pm on Friday 30 June 2025.
This was surprising as (1) there was no preso or webinar, just a straight drop of the 4E (2) it was as late as they come - after trading hours on the last day of reporting season?
Looked at FY2024, and it seems this is normal for BOT - last day drop, no preso (there was a webinar 2 weeks later to update on Sofdra). This being my first year as a BOT investor, is this normal, what I would call "tardiness"??
Discl: Held IRL and in SM
Had a quick glance through the BOT Cannacord Genuity Preso which was released on 12 Aug 2025. It was a good summary refresher of the BOT story for me being relatively new to BOT.
What caught my eye, though, was the few slides on the "fulfilment platform" - see extracts below.
- It was interesting to see the re-positioning of the fulfilment platform as a means to improve GTN yield, expediting fulfilment etc.
- There were 18 content slides in the pack. The fulfilment platform appeared in 5 of the 18 slides, about 27% of the pack - a very decent amount of airtime, I thought.
- This positioning is almost exactly how Matt O'Callahan positioned the platform in the SM meeting - how Matt described it, and how it is positioned in this slide pack makes total sense to me - its a differentiator and will make a difference, but there is no "formal" or tangible" dependence on it to drive growth/uptake
- Quite a sharp contrast to the deafening slience on the platform in the last 2 updates, starting with "The Nightmare"
Given this airtime, this does not sound like a capability that is dead/shafted/pushed to the side ... could this be the start of the platform revival instead, I wonder?
Discl: Held IRL and in SM





Am methodically working through all the posts on BOT since "The Nightmare" of early July to take stock and review my conviction on BOT and action to take. There are lots and lots of very valuable insights ...
Upfront apologies for what feels like a dumb question to me, being rather new to BOT, but it keeps popping up in my head as I work through the posts on BOT:
"What is the utopia target or targets, implicitly or explicitly, that the market is expecting from BOT which caused the huge disappointment following the July "The Nightmare" announcement ie, oh crap, given current trajectory, I think target x is going to be missed/delayed/short ...
Is it $100m top line revenue in CY2026? Or cash flow positive in CY2026? Both? Or something else? I had $100 top line revenue in CY2026 as a goal that I think popped up in the Matt SM interview.
The reason for asking is that it feels like a lot of the analysis seems to be trying to establish if BOT can still get to "something" but I am not clear what that "something" is.
Or should I just ignore that "something" and work bottom up to determine if the investment case still makes sense, given current trajectory, which is how I framed my thesis. Sofdra is going to change lives, there is a huge TAM, and BOT is well positioned to go after that TAM in the coming months/years ... onward and upward?
Discl: Held IRL and in SM
$BOT held a short 4C-focused webinar, keeping strictly to reporting on the quarter.
There were really four big takeaways for me:
Sales and Marketing
The expansion of the Sales Force from 27 to 33 and then 50 (+17 training in 1Q FY26, selling from 2Q FY26), reps is being funded through a reallocation of the overall sales and marketing budget (whatever that was). So management are saying that it isn't leading to a net cost increase.
As I have commented earlier, Howie reiterated that this is rational resource allocation, given how easy it is proving to "activate" physicans. He said that adding more reps is going to be the fastest way to get to breakeven, so they're doing that.
We also said that the digital channel is working, but by inference, achieves a low ROI.
So, they are working as fast as they can to reach all (or 90%) of the 4,000 - 5,000 prescribinbg Derms.
In terms of my valuation, that a positive, as it means I might be able to dial back the high-cost sensitivities I ran. (I will look at that later today, once I've gone through the 4C).
Cash Burn Will Stablise Quickly
In addition to reallocating funds within the sales and marketing budget, the made a big splash in 4Q on buying enought active agent (API) to support iunventory for 1H FY26. In fact, I think they said they won't be buying an more API in 1K FY26. That will help stablise cash outflows for the next two quarters, all the while we'll see cash receipts ramping quickly.
They have the Cash to Get to Breakeven and Won't need to Raise Capital
Vince put the CFO on the spot to answer that question. The CFO was careful with his words and didn't say they won't raise capital, but implied they wouldn't "if they hit their revenue targets".
I mean, what else can a CFO say. He's the numbers guy and clearly they have a set of revenue and cost budgets that get them to profitability without further capital. But he doesn't control the operation or the revenue, so I guess a cautious CFO would only be able to say what he said. That's as good as we can hope for.
100% Focus on Sofdra
There were clearly questions about development of other products and licensing. Vince made it clear that spending on other product development is on ice, and that there is some BD on prospecting for products to license in. But he made it clear that management's sole focus is making a success of Sofdra and getting to profitability. Good.
Conclusion
Happy with my analysis last week and over the weekend on this one, and I think this investment now just needs patience so that over each quarter we can see how scripts and GTN trend. These guys seem to have the cost side of the equation under control, and they are clearly focused on wanting to get to profitability as soon as possible.
Reaffirming my HOLD decision from last week.
Disc: Held in RL and SM
In this Straw I set out details of the valuation I posted last night. I know Monday's webinar may well quickly date what I write here. However, it is a line in the sand because it sets out the basis for why I continue to HOLD $BOT. And that was a decision I needed to take today. If Monday brings new information, so be it.
SUMMARY
This Straw presents a valuation analysis of Botanix Pharmaceuticals ($BOT) based on the first six months of SOFDRA sales. Using updated data from the 8th July 2025 company webinar (The “Nightmare on Hyperhidrosis Street”), I built a scenario-based revenue model projecting to FY28 and applied forward P/E multiples to derive a valuation discounted to FY25.
Key components of the model include:
- Market Penetration: Three uptake scenarios (70%, 85%, 100%) based on prescribing dermatologist adoption over 24 months, with consideration of potential GP involvement and expansion of the specialist base over time.
- Refill Dynamics: Volume driven by average new scripts per prescriber and monthly patient churn. Churn is conservatively set at 18.5%, with sensitivities at 16% and 20%.
- Gross-to-Net Revenue: Gross sales per refill is AUD 1,500. A gross-to-net (GTN) ratio of 32% is assumed on average, reflecting early-year deductible effects and expected improvements in reimbursement management.
- Cost Structure:
- Sales & Marketing: Based on sales force headcount and dermatology industry benchmarks (AUD 462k per rep), with sensitivities up to AUD 20 million additional spend.
- COGS: Assumed at 7% of gross refill revenue.
- Other Expenses: Adjusted from the FY25 interim results, net of sales and marketing.
Twelve scenarios are modelled, combining varying assumptions for prescriber activity, churn, GTN, and S&M cost. The base case (P/E = 25) yields a valuation range of $0.22–$0.90, with a central (p50) estimate of $0.35. Even in worst-case scenarios, valuations remain above the current share price of $0.16- $0.18.
Using an alternative methd of applying an M&A revenue multiple of 5x FY28 revenues and discounting back yields valuations of $0.24 to $0.42. (Average is $0.33)
I conclude that despite recent market pessimism, SOFDRA retains strong risk-reward potential, and management has a reasonable timeframe to demonstrate longer-term value generation through platform exploitation and licensing.
INTRODUCTION
My basic approach is to model scenarios for revenue to the end of FY28, estimate the NPAT at that stage and apply a range of P/E ratios at that point, discounting back to end of FY25.
Revenues are driven off modelling total refills per month, using the detailed monthly history provided in the “The Webinar” (aka “Nightmare on Hyperhidrosis Street”, 8th July).
The structure of the analysis is as follows:
1. The Revenue Model
1.1 Market Penetration
1.2 Refill Volumes
1.3 Average Number of Scripts Per Subscriber Per Month
1.4 Patient Churn
1.5 Gross to Net
1.6 So What Revenue Do I Expect
2. The Rest of the Financials – A “Ball Park” Estimate
2.1 Sales & Marketing Expense
2.2 COGS
2.3 Expenses
2.4 Getting to NPAT and EPS
3. Valuation
4. Model Outputs Discussion
4.1 Discussion
4.2 M&A Valuation
5. “A Nightmare on Hyperhidrosis Street 2 – The Revenge of the Applicator”
6. So, What About My Thesis?
------------------------------------------------------------------------------------------------------------
1. The Revenue Model
1.1 Market Penetration
The market is large with some 3.7m seeking treatment in a Dermatologists office out of an estimated market potential of 10m.
The key volume drivers are therefore:
· How many dermatologists (Derms.) are prescribing Sofdra
· Number of new scripts written per month
· How many refills each patient gets
We know there are around 4,000-5,000 Derms. who see patients with PAHh and will therefore assume 4,500 as 100% of the prescribing base.
The market penetration scenario assumptions are:
· Maximum penetration achieved over 24 months
· Penetrations of 70%, 85% and 100% modelled.
This is justified because very rapid penetration (51%) was achieved inless than 6 months. However, ultimate penetrations of 70%, 85% and 100% might at first glance appear unreasonably high. However, there are three further factors to consider:
First, the actual Derm base is 10,000-12,000, so if the product gains market acceptance, there is the possibility that the specialist prescribing base expands.
Second, the experience for the other anticholinergic in the market (Qbrexa) is that over time, some GPs will prescribe refills, or potentially write a script for a patient who has tried the drug but them come off it (for example, at first they couldn’t get the health fund to pay). Apparently, this has been written as acceptable by some health funds (Note: verification of this is required.)
Finally, the upside case (100% of 4,500) also allows for the potential that there are actually 5,000 prescribing dermatologists to begin with.
In short, while 100% penetration is unlikely, there is the potential for the prescriber base to grow over time.
A peak in number of prescribers is assumed to occur in 24 months from launch. The three modelled uptake scenarios are shown below. These scenarios are consistent with the observed fact that in the US dermatology treatments tend to reach plateau sales in the 3rd year.
Exhibit 1: Modelled Prescriber Uptake Scenarios

1.2 Refill volumes
Scenarios are generated for the number of refills issued by month. The assumptions in this model are:
· Existing patients obtain refills, subject to a Monthly Churn Rate (% Churn).
· Active Monthly Prescibers write “n” Scripts per Month
From this simple model, the number of refills in any month is simply:
TRx(n) = Total (Re)fills issued in Month “n” = TRx(n-1) . [1 - % Churn] + NRx(n)
where
NRx(n) = number of new scripts (i.e. new patients, including returning patients) written in the month.
In turn we can find NRx(n) from the Total Number of Prescribers (n) x # Scripts per Subscriber Per month.
So, we have two key variables we now have to understand:
· Number of new Scripts written on average per Prescriber
· % Monthly Churn.
I’ll next look at each of these in turn.
1.3 Average Number of Scripts Per Subscriber Per Month
Here we turn to the data from the first 6 months from The “Webinar”, and perform the analysis shown in Exhibit 2 below:
Exhibit 2: Model Calibration – New Scripts and Churn

Source: The figures in blue are from the “Webinar”.
I’ve estimated the New Scripts in each month (the Churn model is described in the next section). From this, we can calculate how many new scripts were written per Prescriber in each month.
Interestingly, the number started very high, which indicates that early prescribers might have already “warmed up” by having been engaged over the prior 3-6 months as part of the Patient Experience Program. In any event, $BOT presumably had a kernel of super-prescribers and KOLs ready to go at launch.
In previous Straws, we’ve also spoken on this forum about whether a potential “bolus effect” exists. The would be from highly motivated patients aware of the products approval and actively seeking it after launch.
So, the rapid fall-off in the Average Number of Scripts per Prescriber per Month is unsurprising. In fact, we expect it.
A source of error in this analysis is the Churn model leading to an estimate of the number of patient “Lapsing” each month. I’ve played around with different “% Churn" values, and the overall observation is robust.
Scripts per Prescriber per month falls rapidly over the first 6 months, although appear to be levelling off. This is reasonable if the initial population of "super-prescribers" gets diluted by the more general population and/or if the “bolus” effect dissipates rapidly in the early months after launch.
Now, the key question is how this number changes over time.
There is evidence from other drug launched in dermatology, that indicates that the prescribers initially prescribe at a low level, and that this grows by 2-3x over the next 12 months.
Whether this proves to be the case for SOFDRA is one of the big value drivers and uncertainties. At this stage it is unknown.
Note also that I am ignoring the prevalence of the condition at this stage. It doesn’t matter, because the results of the model represent a very low proportion of the prevalent population, so Sofdra will not be limited by the number of patients seeking treatment.
Conservatively, I have generated the following 3 scenarios, which I hold as independent to the number of prescribers:
Exhibit 3: Scenarios for New Scripts Per Subscriber Per Month

I have clearly excluded the scenario that the product “flops”, and clinicians reduce their prescribing over time. This scenario cannot be ruled out, and could occur under two situations.
· Clinical Data doesn’t support continued use (for whatever reason)
· A superior treatment emerges.
I’ve ruled out the first case because of the clinical trial data published by the JAAD, but also the experience in Japan, which showed consistent growth over the first three years in the market. While the product is only partially effective, it appears to be well tolerated and delivers a sufficient benefit to be meaningful to a reasonable proportion of patients who try it.
At this stage, it is unclear whether a superior product will emerge in the near future.
Note: This sensitivity most significantly impacts the valuation.
1.4 % Monthly Churn
The Webinar covered a lot of information about adherence and number of refills. I’ve developed a basic monthly churn model, simply because it is the easiest way to fit the data provided for the first 6 months, and then to project forward.
If 18.5% of patients churn off the drug each month and don’t return, we get the following profile:
· 3.46 total fills from February to June (i.e.2.46 refills)
· Only 11% of patients remain at the end of the first year
· 4.94 fills over the first year
The first point fits the data presented by Howie in the webinar.
On the second point, no-one knows how many patients will come back for another script at the start of the second year. However, 11% seems a conservative approach. Perhaps more will if a significant proportion of reimbursed patients perceive value from the product. So, there is a significant potential upside that I have not considered, as I am choosing a cautious approach in absence of data.
The % Monthly Churn model is flawed. For example, we know a proportion of patients are not going for auto-refills, and are maybe only trying 1 or 2 refills, before abandoning the treatment. However, I am basically comfortable with the model as a rough estimate, given that:
1. It predicts well the average number of refills for the February patients over a 5-month period
2. It aligns with managements enthusiasm that the product is performing well above the norms for dermatological products, which have of an average of 2 fills per patient (i.e., only 1 refill).
I will run two sensitivities on this parameter at % Churn levels of 16% and 20%, noting that at a 20% monthly churn, only 9% of patients are still using the product in the 12th month after first prescription.
This is an area of high uncertainty, and based on performance over the first 6 months and management’s statement about the February Scripts, there is a possible material upside risk to this factor. Rather than introduce further model complexities, this will be something to revisit over time.
1.5 Gross-To-Net (GTN)
$BOT appear to be achieving Gross Sales of AUD1,500 per refill. So with modelling the volume of scripts well-defined, the next big parameter is GTN, in order to achieve net revenue. Management believe they will ultimately achieve a GTN of 30% to 40%.
The exit rate for June was 23%, improving at about 2% per month. Given that Q1 and Q2 are the high deductible season, recovery to the mid-range seems likely.
Secondly, I expect management to tighten the copay policy in Year 2, and also for optimisation of Pre-authorisation of Scripts over time to improve GTN over time.
Analysis from studies of other drugs in sectors like derm. shows that Q1 and Q2 are typically hit by the high-deductible period, with stable revenues in Q3 and Q4,
I have therefore derived the following assumptions based on other studies (note: at this stage $BOT management haven’t said much about this):
· GTN continues to improve at 2% per month, reaching 35% by end of calendar 2025.
· Thereafter, every year, there is a Q1 hit to 72.5% of the Q4 value, and in Q2 88% of the Q4 value, with full recovery by Q3.
We don’t yet know what the Q1 and Q2 annual deductible hits will be. However, the chosen values seem reasonable given experience elsewhere.
The net effect of the 35% assumption, and the annual resets lead to an average annual GTN of 32%. (Note: this is down from my original valuation of 50% - a bit hit to value!)
I have not run any scenarios or sensitivities on GTN. Who knows, perhaps average annual GTN is only 28% or maybe it is 36% - these are now relatively small uncertainties compared with others discussed here! So, I’ll settle with 32% as a reasonably conservative but not unduly pessimistic number. Exhibit 4 shows the GTN over time.
Exhibit 4 GTN over Time

This concludes the revenue model assumptions.
1.6 So What Revenues Do I Expect
According to my model, Sofdra will generate peak revenues of anywhere between $AUD137 and AU$240m by FY28 (or US$90m – US$160m).
That’s very materially down from upside cases I was projecting of anywhere from US$200m to US$600m only a few months ago. (Sad face emoji)
Of course, it is possible that in every assumption I’ve made in this model I’ve suffer from a negative bias induced by the “Nightmare …” and there are certainly upsides I’ve chosen not to consider, particularly around GTN optimisation and, more materially, increasing prescription rates over time.
But rather than “fudge” my model, I’ll run with what it's telling me and – if warranted over time – I'll make adjustments in the light of evidence.
2. THE REST OF THE FINANCIALS
With a high range of uncertainty around the revenue model, I have kept the rest of the financial modelling simple. I’ve also not spent any time trying to get a sensible number for FY25 simply because it is a transition year, with several non-recurring factors:
· Platform build
· Launch preparation
· Onboarding of Sales and Marketing Staff
· Launch inventory build
I want to emphasise this because I will not judge this model by how well it predicts the FY25 Full Year result. I’ve spent zero effort trying to do that because it has no bearing on the company value in the medium term - even though it may well drive the market.
The major uncertainty is the spend on Sales and Marketing. So my approach here, is to take the Expenses from the 1H FY25 Accounts, back out the Sales and Marketing element, and build a simple sales and marketing cost model.
2.1 Sales and Marketing Expense
I will estimate the total Sales and Marketing Expense as follows:
S&M Expense = Sales Force Headcount x Benchmark value
This is a crude but well-established method in pharma to derive total S&M Expense from the size of the field force, with the benchmark picking up all related and overhead costs.
Reasonable benchmarks in Dermatology are anywhere from $USD 300 k per FTE to $500 k per FTE, which turns into AUD 462 k to AUD 770 k.
We know that $BOT have hired an “A” team of derma industry veterans. And “first product” businesses usually pay over the odds. This will be offset by the fact that some of the expenses covered by the benchmark are already “hidden” in other lines of the Accounts – given the AASB/IRFS model applied in Australia.
Therefore, the approach to be followed is as follows:
· Assume AUD 462 k per FTE
· Run high case sensitivities of AUD$10m, $15m, and $20m (for a 50 strong field force, these sensitivities are equivalent to AUD200k, 300k and 400k per head – so they should cover the potential outcomes).
These sensitivities are also important because we don’t know how much digital marketing spend has been thrown at the business.
Most of the platform build will be included in the 1H FY25 accounts, So we don’t need to worry about that. However, it is clear to me from the Webinar that $BOT are not yet spending big on digital marketing, and as I’ve written previously, that they are seeing the highest ROI on investing in the good old door-knocking salesforce.
Why is this the case? Well, it appears the physicians are easily “activated.” So rational resource allocation is to get your reps in front of all the 4,000-5,000 target derms. asap! Which is what management appear to be doing.
For the model, Sales and marketing is built up as follows:
· FY25: 27 Reps (FY25 is not refined as it's immaterial to valuation)
· FY26: 50 Reps
· FY27: 60 Reps – they go from 90% coverage to expand the base in targeted areas.
And so the Sales & Marketing expense then follows.
2.2 COGS
I’ve assumed a flat 7% of Gross Refill Value is assumed. i.e., 0.07 x AUD1500 = AUD105 per refill
This is on the high side to allow for Tariff impacts (assuming Tarrifs apply to COGS and not Sales!)
An error in the model is that inventory needs to be made 3-6 months ahead of sales, but this is not material given all the other assumptions, so I’ve ignore working capital.
2.3 Total Expenses
Expenses are estimated as follows:
· The expense base at 1H FY25 Accounts (4D) as starting point: AUD 32m x 2 = AUD 64m
· Strip out Sales and Marketing, so it doesn’t get double-counted: -AUD 17.7m
· Expense Base = AUD 47m + Sales and Marketing.
I’ve assumed interest is included in here, and may have under-estimated charges for the expanded debt facility.
2.4 Getting to NPAT and EPS
PBT = Net Revenue – COGS – Expenses
Tax rate assumed at 25%, as there will be benefit from carried forward tax losses.
NPAT = PBT * (1-Tax)
Shares on Issue: Management are using a fair amount of share-based compensation, so I assume 3% dilution p,a,
3. VALUATION
The model generates FY28 NPAT for 12 scenarios, combining the various factors covered.
P/E Ratio – This is the second big driver for the change to my valuation. The change in this valuation over my pervious valuation is that SOFDRA does not appear likely to be a blockbuster. It looks like it will be moderately successful and reaches maturity in FY28, and is not rolled out beyond the US. (The economics are not attractive.)
Growth from FY28 onwards will then depend on whether – over the next three years (not tomorrow!) – management can bring other undervalued dermatology drugs onto the platform.
I’m not sure they’ll succeed and so the P/E ratio scenarios I will apply in FY28 are 20, 25 and 30.
If you think $BOT is “Sofrda and done”, then eventually it will get bought out at some multiple.
So, I’ve taken FY28 EPS and discounted back for 3 years to end of FY25 at10%
Bingo.
This is still betting on management experience and skill in dermatology, and it gives them a reasonable time horizon to either do platform deals or licence in new molecules. If I didn’t believe in management, then P/E scenarios of 15 and 20 would probably be more appropriate. This risk is not explicitly modelled, but that’s because I believe management will find a way to create more value over time.
The detailed inputs and key outputs are listed in Exhibit 5.
Exhibit 5: Model Scenarios and Outputs

The table above shows the outputs from the various scenarios. I’ve not really had the time to think about the scenarios probabilistically, but if I had, the distribution of valuations would be as shown in the Exhibit 6.
Exhibit 6: $BOT Valuation Results

At my refence P/E of 25, I get a valuation range using my usual p50% (p10% - p90%) notation, of $0.35 ($0.22 - $0.90) in roundabout terms.
At my p50% level, the range generated by my P/E values (20, 25, 30) are $0.27 to $0.41
My conclusion is that the market has indeed over-reacted to the “Nightmare on Hyperhidrosis Street”. Even in my lowest case analysis, I can’t get below $0.17. And yet that’s where we are today at $0.16 to $0.18.
While my previous very bullish view on $BOT has been materially deflated (sigh), I think the market has got this one wrong. Standing here today, you’d probably need to give me $0.60-$0.70 to get me to part with my shares.
4. Discussion of Valuation and Model Outcomes
4.1 Discussion
Depending on how you compose your scenarios, you can generate either more valuation results at the low end or more at the high end of Exhibit 6.
So, picking a number is indeed a fools game. I’m not sure of the value of doing more analysis on this, simply because the spread of valuations starts squarely at today’s market price and are solidly risked to the upside. IF YOU BELIEVE MY ASSUMPTIONS.
It is true that I could easily generate valuations down to $0.10 or lower, but equally, I can easily still get valuations north of $1.00 – in both cases using reasonable assumptions.
But based on what I believe to be reasonable assumptions, I am a solid HOLD on $BOT given by 4% RL position.
4.2 M&A Valuation
If we assume that by FY27 it becomes clear that $BOT is nothing other than “US Sofdra and Done”, then it won’t make sense as an ongoing entity and will get acquired.
To test the valuation, I’ll apply a modest 5 x FY28 revenues, and discount back.
Doing this I get a range of valuations of $0.24 to $0.42. Funnily enought, the midpoint of $0.33 is eerily close to my bottom-up $0.35 p50% at P/E = 25. (Honest, I haven't had time to fudge the models!)
5. “A Nightmare on Hyperhidrosis Street 2 – The Revenge of the Applicator”
So, why another “comprehensive” webinar on Monday?
I think management HAVE to do this because the 4C is doing to drop on Monday. Revenue and Cash will be bugger all, and cash burn will be scarey. And so management has to help the market make sense of the cost base. If they don’t do that, half the analysts will predict that $BOT runs out of money pretty soon.
And I don’t think they will run out of cash. For example, I’ve plotted the financials below for one of my more central case scenarios in Exhibit 7. $BOT can get close to breakeven in FY26 and is strongly cash generative in FY27.
Exhibit 7: Modelled $BOT Financials (Scenario 6)

Even in the case where I’ve layered on $20m of excess sales and marketing costs, with the lowest monthly prescription case (Scenario 12), there’s probably enough liquidity to get through to positive cash flow in FY27, just.
Exhibit 8: Modelled $BOT Financials inHigh Cash Burn / Lower Revenue Case (Scenario 12)

Of course, I’m also hoping for some more insights about rollout. After all, there’s been another 4 weeks of data, so hopefully there’ll be an update on scripts and prescribers.
6. So. What about My Thesis?
The whole point of doing all this work was find out if my investment thesis is intact or not. And?
My investment in $BOT was initially predicated on the view that the market was seriously mis-pricing development and execution risk. Development risk mispriced, because we knew the product works based on experience in Japan and the promising US clinical trial data (now published in JAAD). Execution risk overblown because 1) there is huge unmet need in the market, 2) the management team have a strong track record in dermatology launches and 3) the existing anticholinergic product in the market has well-defined deficiencies, and is being managed by a lightweight company trying to juggle multiple products.
I bought $BOT between $0.325 and $0.47 in the belief that this business was worth anywhere from $1.00 to $2.00.
Wind forward to today, and while physicians are getting onboard, prescription rates are underwhelming, and conversion to net revenue is less than (I) expected. Added to that, the company is rapidly scaling up sales and marketing spending.
So, the market is in the doldrums, seeing this business as worth $0.16 - $0.18. But I think it would be worth anywhere from $0.20 to $0.90. That’s a serious haircut to what I thought, but still an interesting investment. And it is early days.
So, my thesis while seriously diminished, is not broken. I’m happy to see how this story continues to unfold.
At this stage, I’m not sweating.
Disc: Held in RL(4%) and SM
Heads-up $BOT holders, management are holding a "webinar to provide a comprehensive update on the Company’s Quarterly Activity Report and 4c Cash Flow Report. The webinar will include an update on the increasing launch momentum for Sofdra™ (sofpironium) topical gel, 12.45% and how Botanix’s cash position will support Sofdra through to profitability."
Monday 28th July, 9am.
Be there or be square!!
Given the SP reaction to the last sxxx show, this is hardly a surprise**. But it probably an indication that management believe the market reaction to the result was badly wrong. And they will take steps to give assurances that they are not going to run out of money before geting to profitability.
(** Actually, I listen back to the recording several times over, and there was a lot of good information provided by Howie, which I've been able to build into my model.)
My Valuation Update
I have been beavering away on my detailed digestion of the last webinar. Now the pressure is on for me to complete the work today, and during trading tomorrow to decide my investment strategy.
What is emerging from my analysis so far, is that there are a wide range of scenarios for how Sofdra will play out. Certainly, many aspects of the first 5-6 months data are promising. One example is the rapid penetration of the key prescriber base, which really does look like a best-practice launch rollout.
Less impressive are the scripts per prescriber. How this evolves is a key value driver and a key uncertainty. For sure, it is perfectly normal that prescribing frequency is low in the early months, and it often picks up through the 6-18 month timeframe, based on market response and clinical feedback and competition (low here).
The market was clearly shocked by the GTN numbers. My research on this indicates that in the second half, we will see GTN increase significantly, and this will drive net revenues significantly.
Apologies for the teaser, but I am still working through my various scenarios. I'm still seeing plausible cases that hit my current valuation, but the risk-reward has shift downwards, significantly. And based on the data so far, there are plausible scenarios where this business doesn't amount to anything.
So my investment decision is going to come down to how I assess the various scenarios (sorry, that's a statement of the blindingly obvious). I'm flipping at the moment between viewing that the market got this about right. ($0.2 - $0.3, risked valuation) through to no, no, no, its a complete over-reaction ($0.6 to $1.0). So, I better get my head down!
At this stage, I am on the fence as to whether my investment thesis is intact or not.
One thing I do tend to agree with in the webinar announcement,... Sofdra sales ramp appears highly likely to get to cash flow positive based on existing cash reserves. So that is one source of anxiety I am feeling better about. But only one.
Have had some time to analyse the presentation and come up with the best way to monitor upcoming progress,
I feel The simplest metric to track is the net increase in users per month after the churn along with the gross to net. This was reported as the Tx / month.
Short version, it probably still isn't as bad as it looked and has been sold down heavily, won't be a 6 month success story but even on the lower end case things will be ok in 12-18 months time and they could be close to a profit, main takeaway is cash on hand after the raise and factoring in the debt facility a raise shouldnt be needed.
Welcome anyone's thoughts/comments on this.

As noted in a separate post, when you remove the free units and factor in the PA units that get retrospective approval it isn't as bad as it looks. The high deductible dip in the given example didn't seem to be as bad in the year after the launch but that remains to be seen, I have put a dip in year 1 post launch but not year 2.

Another thing to note is the adherance rates, it was 79% overall and 95% for people in the auto refills,

The 79% adherance rate on the surface looks low but it is most likely related to the free units being shipped each month (mid 20%, assuming these aren't eligible for refills and these account for most of the discontinuing users.
If this assessment is correct, the actual adherence rate is still quite reasonable.
All this being said below are 3 cases to consider, both with an example for GTN of 30 and 35.
All have annual opex of 80m to allow for the extra reps, COGS as noted below, and the bodor royalty is 5% of net sales.
*need to confirm the opex in the quarterly, might still be hard to tell with the foray into digital that didn't go well but should be a good indicator at least.
Case 1:
Maintain the net addition of 1150 users on average per month as has been the case so far, GTN tracks to 33%, with a dip being overserved in high deductible season.
Not sure if the bigger table will be readable, but COH bottoms out at 70m, with a cashflow positive month in June 2026.


Case 1b:
GTN peaks at 30 with 1150 / month

Case 2:
Net Addition of users gets up to 1500 / month with the addition of more sales reps and an S curve effect with prescribers becoming more productive.
Cashflow positive August 2026, COH bottoms at 60mil.

Case 2b: GTN peaks at 30 with 1500 / month

Case 3:
Growth underwhelms and has peaked at 1150, continues to add at 750 / month with additional reps.

Case 3b: GTN peaks at 30 with 750 / month

This is just a start in re-understanding BOT…
I am starting with understanding GTN both % and $. I think we can learn something useful from yesterdays presentation about this, unlike the other important disappointment regarding patient and prescription numbers which has a lot of fog to lift.
So what is the Gross Sales per script.
Matt had indicated that the Gross sale price was between US$500 and US$1,000 but closer to US$1,000. So US$750 to US$1,000
Based on the presentation the Gross Sales per customer is US$975, this is an average but one with very little variability – suspicious? maybe, or just a fact from initial launch and single distribution channel (welcome input on how these prices are agreed with insurers from anyone who knows, or there is something wrong with my calc).
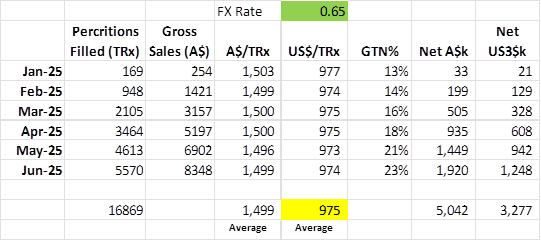
So what is the GTN% profile and likely Net.
Initially Investors were told to expect approximately US$450 per prescription as net revenue. This was talked back recently to US$400, but analyst EUROZ still had the US$450 per prescription back in May.
Assuming they knew the gross price of US$975 at the time (which is reasonable given insurer negotiations were mostly done), that would be an average GTN% of 46.2% at US$450 net sales. They would have taken into account some element of deductibles would eat into a fully reimbursed margin, so we can probably assume that Fully Reimbursed GTN% is over 46.2%.
The subsequent talk down to US$400 (41.0% GTN), was probably in response to early indication on higher deductible impact and the sales approach effect of Non-Reimbursed sales or sales prior to authorisation ending up not being authorised.
The presentation sets out the types of reimbursement status that impact GTN. It also shows the weight but not what the GTN% of each group was. So here is my attempt to unravel the confusion on what each group represents in terms of GTN:
Full Reimbursed Units: These are full margin, highest GTN% sales which should only be offset by patient rebates (co-pay coverage), which on slide 10 is 50%. I am assuming the “Managed Care Rebates” of 21% are not included or any wholesale costs due to cutting that out. So may be 50% GTN% for these sales.
High-Deductible Units: BOT will cover the deductible for patients, which added to the other out of pocket coverage is the total price. Hence, I expect these are all 0% GTN%
PA Pending Units: This is messy, BOT would get nothing until they receive authorization, which they say 70% get approved, so 30% they get nothing ever and presumably stop (or become non-reimbursed). The rest may start to be reimbursable later, I assume those issued prior to authorisation don’t get reimbursed later. So at best they get 70% of the Full Reimbursed, but with a delayed – so I don’t think we see revenue until later and at a lower average GTN%.
Non-Reimbursed Units: They should just call these “Free Samples”…
So let’s assume a 50% GTN% for only Fully Reimbursed and zero for everything else and see how it lines up with the reported GTN%. Below you will see it starts on the money but a gap opens up to be 3-4% low by June.
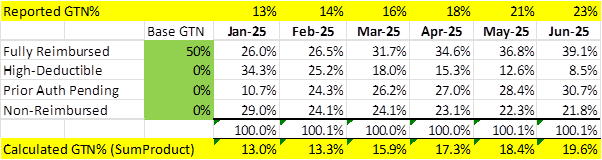
So I played around with the % for Prior Authorisation Pending and delayed the impact by a few months and got a good fit at 2 months delay and average GTN% of 20%.

While not conclusive, it indicates to me that the GTN% for Fully Reimbursed sales is likely around 50% (from both this calc and the inference from the US$450 expected net on extrapolated gross US$975).
Given it is very early days, I think we can expect the weight of PA Pending and Non-Reimbursed to drop steadily as a total %, also the High deductible will be seasonal. So GTN% should improve, but how quickly and to what steady state I am unsure. To get to a US$400 net per script, they would need to get to an average of just over 80% of scripts being Fully Reimbursed as an example.
Conclusion
1. I am comfortable that GTN% will improve steadily, the rate of growth in patients will impact this significantly. Faster growth will hold GTN% back given the low base, but as the patient base matures we will see much higher Fully Reimbursed % and so higher GTN%
2. A 50% GTN% for Fully Reimbursed scrips seems to be reasonable given the current distribution model. Hence this is a cap, but a high cap, so we may see 30-40% GTN% in the next year, but over 40% will be a way off and challenging.
3. It is also clear that as much as we as investors are struggling to understand the economics of the business, those in the company are also challenged…
Disc: I own RL+SM
Sums it up this was chaotic and 50% falls doesn’t make sense. Oversold territory.
https://stocksdownunder.com/botanix-pharmaceuticals-asxbot-crash/
The language of this is interesting and positive sounding ...

Discl: Held IRL and in SM
Some research from via the IHHS, FWIW. Nb: I am yet to read all links, myself.
From the IHHS website: https://www.sweathelp.org/home/news-blog/562-pearls-from-the-latest-hyperhidrosis-research.html
Hot Topics in 2025: Summer School Is in Session!
School’s out, but we’re still hitting the science pages! Consider this your hyperhidrosis journal club—bringing you pearls from the latest published research on hyperhidrosis.
Sofdra™
So far this year, key articles have been published in medical journals about the newest hyperhidrosis treatment on the market: Sofdra—a topical gel also known as sofpironium bromide.
Importantly, three of our co-founders and members of the Board of Directors published pooled safety and efficacy data on Sofdra in the Journal of the American Academy of Dermatology, based on two randomized, double-blind, controlled clinical trials. The combined trials included 353 people in the treatment groups and 348 in the control (placebo) groups. Patients as young as nine years old participated, but most were adults who had suffered from underarm excessive sweating for about 14 years. Analysis of the combined results, say the authors, shows “statistically significant and clinically meaningful” improvements in underarm excessive sweating (primary axillary hyperhidrosis) from Sofdra treatment, based on both patient-reported outcomes and objective sweat measurements.
Using a recognized scale for measuring excessive sweating severity (the Hyperhidrosis Disease Severity Scale), the data shows that more than 56% of patients had a 2-point improvement in their underarm sweating severity when using Sofdra, compared to 37% using the placebo. In terms of measured sweat volume (how much sweat output a person had), patients using Sofdra experienced a 138mg decrease in sweating, while those using the placebo decreased their sweating by 114mg. The authors note that, while the placebo (a gel without Sofdra’s active ingredient) gave patients some results in terms of patient-reported outcomes and reduced sweating, this was expected because the gel “has emollient properties and can affect skin function. Additionally, patients with hyperhidrosis experience anxiety about their sweating, and this anxiety could trigger further sweating whereas the hope of receiving treatment could reduce anxiety and anxiety-produced sweating.”
No serious side effects from Sofdra were reported. The most common side effects were dry mouth, underarm pain, blurred vision, dilated pupils of the eyes, underarm redness, and swelling and irritation of underarm skin.
In other Sofdra news, in a letter to the editors of the journal Annals of Medicine and Surgery, eight authors reiterate the usefulness of Sofdra to treat underarm excessive sweating noting its targeted nature (localized to the skin of the underarms), its effectiveness, and its more limited side effects (as compared to those of oral medications.) Their call to action is that more people should be made aware of this treatment option and that it should be made more affordable and accessible. Cost effectiveness studies of Sofdra compared to other treatments, they say, could be especially helpful.
Apparently (according to The Australian) US Investment Bank HC Wainwright, have initiated coverage of $BOT at $2.00.
HC Wainwright are a leading deal maker in the US in the small and mid-cap space, however, their SP recommendations have a reputation for being aggressive. Biotech is one of their focused sectors. Take from that what you will.
(Patiently waiting for the next sales update...)
Disc: Held
Botanix Signs Debt Facility with Kreos Capital
Key highlights
• Botanix has worked with Kreos Capital to establish a ~A$48 million (US$30 million) debt facility
• The new debt facility will be available to Botanix for working capital purposes for the
commercialisation of Sofdra™, as well as other platform expansion opportunities
• Following the recent A$40M capital raising and cash on hand at the end of the last quarter
(~A$28M)
So now there will be no need for a credit raising and therefore no dilution to existing shareholders, but it also means that Botanix must be moving a reasonable amount of stock as Kreos would have had a look at the books and liked what they saw.
Im not sure if this update from Simply Wall St has had an effect on BOT share price today. Down circa 5% this morning.
I can't see any further detail on the reasoning.

"$BOT today announced that Mr Matthew Callahan has stepped down as a Director, effective immediately, to attend to a medical issue. The Board thanks Matt for his significant contributions to the Company’s evolution to date. Botanix is now a well-resourced, sustainable and rapidly growing commercial organization. We wish him a very speedy recovery, and both the Board and Matt look forward to his return."
This is sad news. @Strawman, Matt's been generous giving us his time over the last couple of years. If you have his email, perhaps consider wishing him all the best from the Community?
As I expected. here's the full text. They'll try and argue that the same product is not available elsewhere. We'll need to see the text of the EO to see if that cuts it!
Botanix responds to US pharmaceutical pricing announcement
Philadelphia PA and Phoenix AZ 12 May 2025:
Clinical dermatology company, Botanix Pharmaceuticals Ltd (ASX: BOT, “Botanix” or “the Company”), is responding to the reported announcement by President Trump on Telegram of a proposed executive order in relation to “Prescription Drug and Pharmaceutical Prices” (“US Announcement”).
Botanix is aware from the US Announcement that Mr Trump is proposing to issue an executive order that is focused on reducing costs of pharmaceuticals by instituting a “most favored nation’s policy” whereby the US will pay the same price as the nation that pays the lowest price for a drug, anywhere in the world.
Botanix obviously has not seen the executive order the subject of the US Announcement, but based on the information in the US Announcement makes the following comments:
• SofdraTM (sofpironium) topical gel, 12.45% is only approved in the USA and is not marketed in any other jurisdiction worldwide by Botanix or any other party;
• EcclockTM gel 5% is a product that contains sofpironium bromide which is marketed in Japan by Botanix’s partner, Kaken Pharmaceutical Company, however it is a different concentration and formulation than Sofdra, and was tested in different clinical studies and populations than Sofdra; and
• Neither Ecclock nor any other 5% concentration of sofpironium bromide gel are approved for sale in the USA and Sofdra is also not approved for sale in Japan.
Based on the limited information currently available in relation to the US Announcement and in light of the above, Botanix does not consider that it is likely that it will be subject to price reductions based on sales of Sofdra outside the USA, as there are none. Botanix will review the proposed executive order when it becomes available.
Release authorised by Vince Ippolito Executive Chairman
BOT is down 16% this morning. Seems as though the following breaking news is the issue:
Trump Seeks to Align US Drug Costs With Cheapest Ones Abroad
Looks like Howy's bus to Rortsville has just got a puncture. Will take some time to figure out what are the implications of all this, but not good news for BOT, NEU and thousands of other US sales dependent drug companies.
Some notes below on competitor products to Sofdra, potentially good options, but look to most likely be limited by insurance coverage due to being classed as cosmetic treatments and also requiring repeat dermatologist trips for application.
Not Thesis breakers but I don't want to see anything coming up that will be nibbling away at the patient base with a more accessible, more effective solution.
- Brella Sweat Patch
This is being put out by a private entity (CANDESANT)so not too much data on it, they raised $35m USD in funding to advance commercialisation in 2023, requires a Dermatologist trip and looks to cost about $350-550 USD to have it done, results similar to botox and is good for about 3-4 months. Considered cosmetic so limited insurance coverage.
How it works:
The Brella SweatControl Patch consists of a sodium sheet with an adhesive backing. When the Patch is applied to the underarm, the sodium comes in contact with the water in your sweat, generating heat. The heat precisely targets your underarm sweat glands to significantly reduce sweat production.
Pros:
- lasts quite a long time as does underarm botox
- don't have to apply it every day
- side affect profile minimal
Cons:
- Expensive every 3-4 months
- Requires a dermatologist office trip each time
- early stages of commercialisation, so note widely available yet.
https://www.mybrella.com/
2: Dermata Xyngari with Daxxify
Dermata have a sponge type material of sorts (Xyngari) which when applied to the skin creates microchannels where whatever the sponge is infused with can go through the top layer (very rough explanation)
They think they can use this with a brand of Botox (Daxxify) for mostly a pain free botox application to the underarms to treat hyperhidrosis. The botox trial for HH have good results but the cost and pain is an issue. Daxxify is a bit more expensive than normal botox but lasts a bit longer.
Dermata are listed NASDAQ: DRMA, tiny market cap.
https://www.dermatarx.com/new-index
They are doing a phase 2A trial, referenced in the link below from Jan 2025.
https://ir.dermatarx.com/news-events/press-releases/detail/75/dermata-and-revance-enter-clinical-trial-collaboration.
Pros:
- lasts as long as normal botox potentially a bit longer
- mostly pain-free application
Cons:
Similar to above, most likely cost and repeated doctor office trips + yet to be commercialised. Assuming phase 2 and 3 trial this could still take a while.
The Dermata offering is interesting by itself but then again if it was going to shoot the lights out for HH one would expect the Brella sweat patch to already be making waves.
The pipeline they have in general is interesting as the Xyngari applicator appears to be effective for multiple things, the HH application looks like a small side effort compared to the other options they are looking at.
Good discussion with Matt just now, which I think clarified a few points which will help us all interpret the newsflow and data over the coming months and reporting periods.
- The target derm market is the 4,000-5,000 medico-derms, and within this they'll focus on the high prescribers. (Implication: so let's not do any modelling based on 11,000-12,000)
- Pure telehealth remains an unknown as to its relative performance, so over future reports I'll be listening carefully to what they are learning. This is really important for future growth, because if the platform is successful, it is an easy route to add value to already commercialised products.
- The early trends are continuing, but don't over-react to indidividual weeks that fall below this trend. Afterall we've already seen on in the initial results (the week of a major dermatology conference).
- Net revenue per refill sounding more like US$400, than US$450 which most are modelling. Possibly due to higher co-pay requirements driven by the requirements of inidividual patients plans.
- Refills at 6-11 p.a. dramatically reduce the number of patients to hit US$100m FY26 sales, versus analyst forecasts and industry normal (1.8 refills). Basically, as other here have modelled, if they continue to add c, 2,000 new patient per month at close to 100% refill rate, there is a LOT of revenue upside. Apart from doing "what ifs" it is too early to narrow the uncertainty, but that's what we have to track in future reports.
- Management and Board pretty much 100% focused at this time on Sofdra. If we see the SP start to recognise a materially higher potential FY26, then entirely possible that they exploit an opportunity to get more capital, to licence in new products, although I note that they favour back-ended deals and are willing to use debt, reserving cash to invest in Sofdra growth.
Everything else pretty consistent with what they've said before, and Matt understandably being very careful not to make new disclosures outside of a proper market release.
So far, $BOT is executing well.
Disc: Held
Matt Callahan on Euroz Hartleys, finding the front Podcast, 28th March 2025.
Bear in mind that this is a Euroz Hartleys sales job. At the same time, it does give some depth to Matts history. His resume does sound extensive.
BOT doesnt come up till approximately 1:06 and does not go very deep on BOT, however worth a listen for a bit of depth on the Matt Callahan story, ahead of his upcoming Strawman interview.
For this brand new episode of Finding the Front we are so fortunate to have biotech and life sciences veteran Matt Callahan on the show. Matt is a Philadelphia based, Australian born life sciences entrepreneur, with a track record of building and exiting numerous life sciences companies in pain, oncology, dermatology, cell therapies, animal health and regenerative medicine.
He starts the show off on the front foot answering the question of Donald Trumps impact going forward on the US Biotech and Life Science sector showcasing his thoughts for aspiring investors.
Matt has accumulated 25 years legal, life sciences, IP and investment management experience and is Founder and Executive Director of ASX listed Botanix Pharmaceuticals, Co-founder and Director of Respirion Pharmaceuticals Pty Ltd and was also a Co-founder of life science companies Dimerix Bioscience Ltd, Mirata Pharmaceuticals LLC and Orthocell Ltd, as well as the Bill Gates backed climate tech company, Rumin8 Pty Ltd.
Matt is such a great communicator and provides some deep insights into being an entrepreneur, the world of life sciences, FDA approval, the phases of a company and distribution. This really is a captivating chat.
Following on from the Cap raise and launch data released below is some Analysis of the path to $200mUSD Annual Revenue,
- Week on Week patient number growth is going at approx 13%, only a small data set
- New prescribers growing well forming a strong base, quick google search shows it looks safe to assume there are at least 10,000 dermatologists active in the US so there should be decent growth in individual prescribers to come.
- Addition of 20% extra sales staff to drive dermatologist adoption.


There is still a pretty wide window for where this can land, the questions to try and answer are:
- How long can they sustain WoW patient growth? This is currrently 2,280 new patients per month as of the 1st week of April.
- Assuming no Bolas affect here and continuing at a WoW growth of 10% (have averaged 13% for the first 9 weeks of launch) it looks pretty safe to assume before the EoFY we could be tracking at new patients per week of 1000 or equivalent to 4,380 / month. If the new sales staff increase uptake this could happen a bit quicker. (10% WoW growth would have this happening end of May)
Weekly New patients acquired:

- If we were to get to this rate of approx 4,300 new patients per month running for 12 months through the next FY with patients already acquired or expected to in this FY this would land at $200m USD revenue for the coming FY.
- This is not factoring in any benefit from the digital campaign and assuming no further growth in user acquisitions per month once we reach that 4300 / month mark.
- Refills are noted as 100% but this most likely will reduce down as we have discussed previously but it sounds like the onus is on the user to cancel rather than to keep them coming. Also a note from the webinar, when the patient is coming up to the end of the 12month mark, SendRX contact the prescribing dermatologist to reming them to arrange for another appointment and renew the scripts, It wasn't mentioned in the webinar but I suppose they could possibly be directed to the telehealth avenue at this point as well.
The target market from the slides was noted to be 3,700,000 patients that have already sought help from a Dermatologist.

- 61,800 concurrent users would give 1.7% market penetration
- As a side note if that level of users was hit from end of next FY, the forward looking revenue assuming no churn issues and not factoring additonal users would be tracking for $333m USD
Table below shows monthly users acquired and cumulative monthly users --> sales.
New users capped at 4380 to factor in any churn and to assume a safe peak monthly acquisition rate.

The Wildcards:
- Acquiring any % of the 6.3m people with HH that have not sought help through a dermatologist
- any small% of these users is a huge benefit to the revenue stream and not accounted for in the figures above.
- Off-label prescription
- Qbrexza has some talk online about have being prescribed off label, hands, feet, groin, head, any off label prescriptions for Sofdra are a benefit that hasn't been factored into the forecast sales, the mechanism is largely the same and some affect should be seen in these areas. There was a question in the Cap raise webinar about if this could potentially be suitable for menopausal women experiencing sweating issues.
$200m USD annual revenue would be inline with the FY2028 forecast from Euroz below and would imply 143m AUD profit. 143, with 1,950m SOI --> 7.3C EPS. pick a PE for final SP.
The Euroz 2029 Operating income of $134m USD --> 206.7m AUD would be approx 10.6c EPS to follow conservatively the year after on similar growth rates the FY2030 or 2031 could be achievable.
Still a wide range for where this can land sales wise but extrapolating out the early numbers and plotting out what looks to be conservative monthly customer acquisitions I will be watching this closely and updating the numbers and trajectory as the data keeps coming. Very encouraged by the early data and to see what the digital channel does once fully activated.
Any input welcome as always, still a shame the launch slipped a few months but good to see it is finally happening with positive signs.

Disc: Held IRL and SM
Might be cleaner in a new straw rather than the cap raise one.
Interesting, especially with the digital not really turned on.
If they can add 100 people per month (seems doable based on the small data sample) and with that data so far being derms only.
On track for rolling annual revenue of $200mUSD by June, at the current run rate $129m USD earnings for FY25/26.
Interesting to see how much this $40 raise can amplify sales when they fine tune the platform.
Didn't pick up anything that turned me off in the webinar, looks like an experience team doing what needs to be done
Although I am still shitty the launch timing was slow and interesting to hear the digital hasn't really started even though it was in for start of March. Or did I not hear that correctly.

BOT | Investor Webinar
Tuesday 15 April at 11am AEST / 9am AWST
https://investorpa.com/announcement-pdf/20250415/127442.pdf
They don't give much notice, but there you go.
Mix are the agency charged with hiring the BOT sales team. It appears BOT are adding extra staff over and above those hired earlier this year.
https://www.linkedin.com/in/cassie-vick-9219859/recent-activity/all/
From Linkedin:
"We hired outstanding talent for the Botanix sales team, including some of the
most credentialed individuals in dermatology, with 79 President Club wins
between them. "In only two months, they have shown the impact a highly motivated team can make
with a promotionally sensitive product. "We are now increasing the sales organization by more than 20%, to meet the high demand from the market."
Howie McKibbon
Chief Executive Officer
Botanix



The link below is to an "article" in The Australian - essentially paid advertising - not uncommon. Based off the Stockhead video posted earlier.
BOT are going hard at this in the last couple of days.
Is this a flag of some description for anyone?
Or is this simply part of the marketing of a company in BOT's current position? ASX300 inclusion, major sales launch etc.
@mikebrisy I know you said that they are acting just as you would expect them to. Does that include this style of "marketing"?
If all is going to plan, surely the numbers will do all the talking, when they arrive. Or does it all require a little song and dance to prep the market?
A quick interview here with Matt Callahan published yesterday:
https://www.sharecafe.com.au/2025/03/12/botanix-transforming-dermatology/
BOT paid for this interview/advert
Howie says: 1.8 fills per patient per year is typical refill rate for dermatological products in the US. BOT aims to get that to 7-12 fills per year for SOFDRA.
There's nothing new here. Howie remains consistent and on message.
https://stockhead.com.au/health/long-shortz-with-botanix-pharmaceuticals-a-new-major-milestone/
Stockhead’s Tylah Tully chats with Botanix Pharmaceuticals (ASX:BOT) CEO Dr Howie McKibbon, to get the short end of the long story on the company’s latest news.
Botanix has recently been included in the ASX 300, which is part of the company’s focus to be the world’s largest and most innovative dermatology company.
Tune in as Howie discusses this new milestone and hear more about their next planned phase for the treatment of primary axillary hyperhydrosis.
This video was developed in collaboration with Botanix Pharmaceuticals, a Stockhead advertiser at the time of publishing.
(My last post disappeared so trying this again.)
Botanix have posted a commercial update marked price sensitive. At first it looked to me like the same presentation from November (not marked price sensitive) so I was curious if there was actually anything further in this one.
Not much, but a couple of details:
- "Sofdra commercial launch underway and sales on target"
- "Refills exceeding target rates" - good to hear. Sounds like patients like the product.
- "No material impact expected from any tariffs on product"
Similar to the CAT post, BOT will be included in the S&P/ASX300 from Mar24!
This is great news as I (and many others!) can finally invest via a super member direct option (yes and index balancing!). I wonder how much the price will run-up in advance though?
Held in SM & RL (largest holding in both)
BOT has this morning released its Appendix 4D and Half year Report
I am running around doing other things just now and havent had a serious look at the numbers.
However one thing that is for me a glaring omission is the results of the PEP.
I was hoping for some figures pertaining to the program conducted with the Hyper Hydrosis society.
I can only assume it was not impressive.
Given that the PEP was essentially preaching to the converted, I find it concerning that we have no numbers around this at all.
Am I being ridiculous?
Hi everyone,
wanted to ask does anyone have a solid understanding of the following:
- US insurance, copay, deductibles and drug tiers
A big part of the thesis with this is that Botanix buy down the copay to zero so essentially the cost of sofdra to the patient is the cost of the initial dermatologist visit or telehealth. However if there is a big deductible cost to deal with for a % of the TAM than this is an issue. Buying down the copay allows them to ship 11 refills to the customer without requiring payment details or further customer contact.
Looks like some tiers of drug bypass any unfulfilled deductible payment requirement, Will sofdra bypass the deductible component based on drug tier and only require the co-pay (Botanix are buying the patient copay down to zero in this case so only the deductible component is relative.
I am not easily able to find if Sofdra does or does not bypass the deductible based on tier and if this doesn't bypass the deductible I wonder what % of the plans would require patients to pay a deductible (assuming they haven't met their deductible limit already for the year which maybe not be that many people considering healthcare costs in the US)
There is no mention of deductibles in any of the commercial launch media, Botanix only mention that they expect $450 gross to net which possible only refers to the % of people that only have the copay portion and there will be no deductible requirements or they are not mentioning that some people will have a large deductible component to consider if they want the treatment.
I am looking further into this, any input would be greatly appreciated as I don't want to stand on a rake so to speak with this.
Hi Everyone,
For the sofdra launch and upcoming half year report from Botanix, I have done some conversions on the Kaken ecclock sales data to try and get an idea of if we launched on par with the Kaken ecclock launch what sort of revenue we might be looking at as a base case.
Unit sales estimate from Euroz table.

kaken 3Q report, target for annual 2,200m Yen this FY

Potential Sofdra sales using Ecclock unit sales adjusted for US population (to factor in some people not being insured I have used a factor of 2 for US pop : Japan Pop when in reality it is 2.6x
Estimating that of the total units of ecclock sold on average there were 2 scripts per individual. Botanix presentations suggest that it is on average a bit less than 2 scripts per individual.
I have worked off an average of 6 repeats per US user and the usual $450 / person and 0.65c AUD - USD.
With the sales team in place I would be hoping for a better launch and more traction than Japan had so thinking these numbers are possibly on the conservative side.

I am thinking this might be close to a base case for the launch considering we have *hopefully* a better sales force, more experience management and the platform factor as well.
Interested to hear everyone's thoughts on this as a base case, possibly reads as a bull case when you look at the numbers though.
Disc held IRL & SM
I've been trying to understand if there are any drivers of the strong $BOT SP action we've seen in recent days.
Clearly, the market is on tenterhooks wondering what disclosures management will make about early sales at the Half Year report, at the end of this month. So my thought was that market watchers are accessing information on early sales or customer responses.
I found the following in my search:
- Report of a Euroz Hartley report on the news-wires
- Some customer reviews on the web (reddit and drugs,com).
TLDR: Euroz Hartley conclude early signs are that Sofdra is effective compared to its alternative with minimal side effects.
My own search of reviews is inconclusive, because I'm not sure how much of what I am reading is real!
As the EH report only came out in the last few days, that might be the source of the increase buying pressure.
--------------------------
1. Report of a Euroz Hartley report on the news-wires
(Not: I have not read the report)
Sales Traction from Botanix Pharmaceuticals' Prescription Gel Launch May Drive Stock Re-rating, Euroz Hartleys Says
February 10, 2025 at 01:53 am EST
(MT Newswires) -- Sales traction from Botanix Pharmaceuticals' (ASX:BOT) full commercial launch of Sofdra, its prescription treatment for excessive underarm sweating, could lead to a potential stock rating above its price target, according to a Monday note by Euroz Harlteys.On Jan. 31, the company said that the full commercial launch of Sofdra is officially underway, with sales professionals set to hit the field within the next week.
Early patient feedback from social media and online sources shows that Sofdra is effective compared to its alternatives, with minimal side effects, with patients receiving full insurance coverage, Euroz said.
The feedback is positive for BOT and raises Euroz's confidence in the company's ongoing commercial rollout.
Euroz maintained Botanix Pharmaceuticals' buy rating and its AU$0.55 price target.
Shares of the company rose 7% at market close.
2. Some customer reviews on the web.
I had a look on Reddit and also at user reviews on Drugs.com.
Hardly meaningful samples, and in either case, beware that anyone can write anything!
My assessment: I am suspicious of the Drugs.com reviews, which are all 10/10 and unreservedly positive. This is inconsistent with the data from the clincal trials. There are also several from September, which was prior to the start of the patient experience program, so I don't know where these patients were getting their product from.
Against, the same benchmark, the Reddit reviews look more like I was expecting to see.
I will continue to monitor these and other channels, but I can't independently either vouch for or contradict the Euroz Hartley assessment. There are other sources including various closed Facebook Groups as well as Tic Tok, that I have not looked at yet. Can't see much on either X or Insta.
2.1 Reddit
Review 1
"I’m a week or so into using it.
Sadly it’s not proving to be a silver bullet for me. Definitely minimizes it, but have had several breakthrough incidents thus far when body temp increases or I consume caffeine. Roughly the same effect as Qbrexza wipes were for me.
Onto DermaDry, which I grabbed during their Black Friday sale. Still on sale now, at 25% off."
Review 2
"SOFDRA is by far the best thing one can try. whichever folks or company has created this solution needs to be thanked a million. Thankyou Thankyou Thankyou"
Review 3
"I sweat a lot so it’s hard to tell but maybe a 10-20% reduction so far with no noticeable side effects. This is my third night applying Sofdra."
Review 4
"I have done 3 Sofdra applications so far. I think I’m noticing a decrease in underarm sweating, but not significant. Hopeful it’ll continue decreasing. I have been getting headaches all week. Not sure if that’s related or a coincidence."
2.2 Drugs.com
20 reviews, suspiciously all 10/10 and very positive.
January 24, 2025
"Had a good experience. No complaints as it works well and just had a slight dry mouth - nothing compared to other treatments. I like that it is easy to carry around too. Cost me nothing and no insurance hassle."
10 / 10
February 10, 2025
"Got Sofdra last week and very happy with it. No side effects for me. Cost zero."
10 / 10
January 4, 2025
"My friend recommended Sofdra to me. She tried it first and liked it. It is my first week. I had some mild dry mouth at first, but nothing else. I like how effective it is and simple to use. I tried a few drugs before: Qbrexza and Drysol. Sofdra is so much better."
10 / 10
January 13, 2025
"Found out about Sofdra from the HH Society website. Have tried it now for a week, and over the last 3 to 5 days, I have noticed a substantial decrease in my HH symptoms. It feels great to finally be able to show my face in public, knowing I don't have my sweat patches anymore."
10 / 10
February 1, 2025
"I used different medication in the past from Drysol, Qbresza, and Botox. Sofdra is really a game changer for me. First, it simply works and with far fewer side effects. Yes, I still have mild dry mouth, but this is nothing compared to redness and dizziness from other medications."
10/ 10
September 8, 2024
"Found out about Sofdra recently. I think it is new to the market. Did some research and tried it. After many years on different treatments, Sofdra is a game changer. Easy application and have no side effects. Very positive impact. Insurance covers it. I will continue to use it. I understand they can mail it to me too, my doctor said."
10 / 10
February 1, 2025
"I used different medication in the past from Drysol, Qbresza, and Botox. Sofdra is really a game changer for me. First, it simply works and with far fewer side effects. Yes, I still have mild dry mouth, but this is nothing compared to redness and dizziness from other medications."
10 / 10
September 8, 2024
"Could not believe how well this worked. As a sufferer of HH, I was over how much Botox would hurt and the time it took to attend appointments. Simple and easy to apply before bed, thankful that insurance covers this product."
10 / 10
September 12, 2024
"Tried a few alternatives over the years, but nothing comes close to the results from this new treatment. Simple to apply, and the reoccurring subscription and delivery were easily covered by insurance. Much happier."
10 / 10
February 6, 2025
"I like it. Works well and costs nothing with insurance. Some slight dry mouth the first few days only. Much better than wipes and Botox. Easy to use, and they delivered it."
10 / 10
January 1, 2025
"I tried Sofdra this week. Worked well for me. Will continue to use it. Insurance covered it."
10 / 10
January 10, 2025
"Found out about Sofdra from Hyperhidrosis Society email. Works really well for me. Had slight dry mouth before going to bed, but nothing compared to Qbresza. Signed up for 6 months."
10 / 10
December 18, 2024
"Tried yesterday. Simple cream but so effective. No side effects. Will continue to use it."
10 / 10
January 20, 2025
"Had a positive experience with Sofdra. It was a while since I did not experience side effects from using other treatments. Good job for the Sofdra team. It cost me nothing, too."
10 / 10
January 6, 2025
"Game changer - I’ve only been using for a week, but I would highly recommend it to anyone struggling with HH - yet to have a breakthrough episode."
10 / 10
September 6, 2024
"Just found it a new treatment. The best. No side effects and really easy to use."
10 / 10
September 6, 2024
"Great experience."
10 / 10
September 8, 2024
"The doctor told me about it. Spoke to my insurance, and it was covered. Really no side effects and worked like magic (easy to apply!). After many years trying different stuff, Sofdra is the way to go."
10 / 10
January 13, 2025
"Was not sure what to think of it, but Sofdra is a big improvement over Qbresza. I have similar effects like Botox, but this is just easier on my body. Good to have something new."
10 / 10
September 20, 2024
"Did some research and tried Sofdra. Tried a number of things before Drysol and Botox. Sofdra works the best and is easy to use."
10 / 10
September 21, 2024
"Super excited about this new novel treatment for underarm sweating!"
10 / 10
Disc: Held in RL and SM
Nice little article in AFR- with “Botanix share price set to double.”
Announcement out today: https://investorpa.com/announcement-pdf/20250117/91967.pdf
He's got to sell down 12.2% of his holdings to pay a tax bill.
I hope it doesnt spook the market too much
Nature of change
Example: on-market trade, off-market trade, exercise of options, issue of securities under dividend reinvestment plan, participation in buy-back
Executive Chair Vince Ippolito recently exercised 8,000,000 Performance Rights (expiring 2 December 2029) into 8,000,000 fully paid ordinary shares and has subsequently sold 3,800,000 fully paid ordinary shares on-market. These transactions have allowed Mr Ippolito to increase his holding in the Company to 15,001,644 fully paid ordinary shares whilst funding the tax obligations for the exercise of the 8,000,000 Performance Rights. The sale of 3,800,000 fully paid ordinary shares represents approximately 12.2% of Mr Ippolito’s fully diluted holding in the Company (including a total of 16,000,000 Performance Rights expiring 2 December 2029).
Mr Ippolito has no plans to sell any additional shares in the medium term other than to satisfy any tax obligations.
Mr Ippolito remains firmly committed to leading Botanix Pharmaceuticals Ltd as the Company transitions into a revenue generating commercial stage pharmaceutical company.
BOT @ Lytham Partners 2025 Investor Healthcare Summit
Matt Callahan talks shop Click here
I didn't garner any ground breaking news from this, however:
Matt discussed the companies "social listening program" which has identified 435,000 HH sufferers discussing HH and its affects on their lives. This snooping in on conversations on social media platforms allows the company to Id potential future customers, put information in front of them and funnel them through to the Telehealth platform. This can be done right at the time that the HH sufferer is looking for information.
It is possible to be made aware of Sofdra, get diagnosed, then have Sofdra shipped and at your door all within 48 hours. As opposed to getting a referral to a Dermatologist via a GP and picking up Sofdra from the Pharmacy, a process that could take months.
While Sofdra was obviously a big focus right now, Matt highlighted the value in the development of the platform.
It seems that Vince is being paid some of his performance rights Click here for announcement
While this is a milestone, decent sales figures are surely what will move the dial. I am supposing that at this point, the stage is being set and while there may be a handful of sales, its probably not that impressive just yet.

It would be nice if they outlined exactly what rights they were. I guess these, as per the AGM:

Someone has started a Facebook group dedicated to BOT as an investment. It's early days. Seems to have started a few days ago. Currently 77 members. It may throw up some interesting discussion, or not. I'll keep an eye out. Nothing to report thus far.
This group is created for Botanix investors or other that can contribute with their expertise in reading report, news or have an interest in Botanix future research! No spamming is allowed, if you want to post give valuable information that is useful for the group members
$BOT have issued a release confirming that initial prescriptions of Sofdra have been delivered via the patient experience program run in conjunction with IHhS.
Their Headlines
• Patient Experience Program underway with first prescriptions issued and Sofdra delivered to patients
• Inventory build and logistics infrastructure testing completed to support full commercial launch in Q1 2025
• Marketing and sales materials developed, tested and refined
• Sales force engaged and preparing for launch meeting in late January, followed by digital program expansion
My Assessment
Basically, this is a communication saying everything is going according to plan.
My view is that the first significant information will come after 1Q 2025, when we see data on the number of prescriptions in what will be a partial quarter after full launch, with hopefully some information on refill rates from early patients.
Still, this is good news, and good that management are keeping the market informed that everything is progressing as planned. Of particular note are 1) that supply chain is prepared and able to flex according to demand and 2) that insurer reimbursement is working as planned. These are both important elements of execution risk showing an initial "green light". Early days, of course, but nonetheless positive.
Disc: Held in RL and SM
Botanix getting highlighted with some big names in the latest Livewire article. Now to see this re-rate where it should actually be. The market is not so forward looking with this one. It’s been a very very long wait for some of us.
My investment thesis in Biotanix (BOT) is focused on the launch of Sofdra in the US. Royalties and sales outside of the US are ignored as are potential later dermatological products in development, but I expect the net impact is unlikely to reduce the value of BOT and likely highly accretive.
Sofdra US Business Thesis:
· Value is likely to be well above current market cap (see Basic Valuation below)
· Management team shows a lot of capabilities of having done this all before and is well motivated and aligned.
· Platform and go to market (DTC) looks to be well prepared, highly efficient so as to reduce friction of onboarding customers and making sales.
· Gross to net on products and arrangements with insurers implies very strong GP providing for high profitability at low penetration levels of a large (10m) market pop.
· Effective in 85% and relative ease or comfort in use is very favourable to alternatives currently in the market, so strong market penetration is quite possible.
· Well cashed up, management expect it has enough to fund the commercial lunch
· IP protection – noting that as a pharmaceutical there is a monopoly on Sofdra and they do not have to compete with copycat products or cheap knockoffs.
Key Risks:
· Approval risk is almost entirely eliminated, but there is still a chance that some efficacy or safety issues may present once in wider use, as is the case for all new drugs.
· Launch execution and market response may not be as strong as expected and the teams previous success not replicated.
· Regulatory risk with the change in government (RFK) could impact the gross to net received or insurers ability to fund or impose additional regulatory costs of sale.
There are still a lot of details to check or confirm but I have confidence that assumptions are reasonable and estimates conservative and I don’t expect any missing details are likely to be thesis breaking, hence I have bought a double weighted opening position given the investment characteristics.
Disc: I own RL
Basic Valuation & Questions (3/12/24)
Back of envelope estimate of potential value based on AU$450 net receipts per monthly script for Sofdra in the US only. Looking at a break-even benchmark (0.1%pen) to see the sales needed, a base scenario (0.9%pen) where sales in the US reach levels comparable to Japan adjusted for population differences. Also, an aggressive bull scenario (11.7%pen) based on Matt’s ballpark market penetration for a leading drug being 30% of those seeking treatment plus 1% of the remaining out of the 10m total sufferers in the US.
Valuation Questions (assumptions that need testing and validating):
· Probability of Base and Bull case outcomes?
· What are the expected manufacturing costs, ie what gross margin do we model for?
· Platform and distribution costs fixed and variable amounts to include?
· Operating costs including sales team and incentives, what to expect?
· What is a reasonable PE or P/S to work off in 3-5 years
· ROW opportunities, growth in Japan is unlikely to add much but direct sales into Europe could be significant, cost and time to do so and likely net revenue and GP%?
· Pipeline opportunities – will absorb free cash short to medium term but how much and what is the revenue and margin opportunity long term?

Conclusion
Subject to a review and refinement of assumptions, but on the basis that they are likely conservative or ballpark reasonable:
Breakeven is probably around a third of the sales achieved in Japan in a market that is three time the size, so should be quite achievable. If sales reach that achieved in Japan adjusted for 3x population then the company is probably a 5x. Above that is in the range of 10-100x.
Hence, it’s quite asymmetric, with the current value achievable on what would be viewed as a disastrous launch. The upside however is astronomical in its possibilities and outstanding at levels of modest success.
Other questions:
· What are the Royalty rates for Japan? Revenue seems very low for 100k patients on a comparable basis to how US looks, suggests royalties of 1-2%, or much lower sales revenue per customer.
· What post launch monitoring and validations are needed (ie Phase 4)?
· What am I missing?
Botanix Pharmaceuticals (BOT) | Bell Potter Healthcare Conference 2024
Matthew Callahan, Executive Director at Botanix Pharmaceuticals (BOT) presents at the Bell Potter Healthcare Conference 2024.
Hiring of sales force team - on track
Telehealth platform (Upscript) - In place and on track
"Payer success continues" (Insurance companies signing/signed up. By all accounts a long and time consuming process. Very important in the scheme of things as it will bring the cost of SOFDRA to zero, for the insured Hyperhidrosis sufferer.
❖ Contracts already signed or currently being signed with Commercial Payers that represent
~80M commercial lives
❖ Finalizing terms on contracts with Commercial Payers representing a further ~80M lives in
the coming weeks
❖ On track to have ~72M Medicaid lives eligible
❖ Contracts signed and being negotiated reflect the expected Payer coverage mix (see chart
previous slide)
❖ Where there are Payer restrictions, those restrictions only limited to those negotiated:
− ensuring that the patient actually has the medical condition per the label; and/or
− the patient confirms they’ve tried an existing product such as Drysol
Patient experience program - On track
Manufacturing - On track
Nb. diagram below refers to the calendar year:

There is not much here that has not been communicated previously.
All in all this is playing out as planned
As discussed here on SM previously, there may not be too much to get excited about until the new calendar year and some sales data from the US, begins to trickle in.
Meanwhile its steady as she goes and stick to the plan/timeline below:

Botanix Pharmaceuticals Quarterly Activity Report and 4C Quarterly Cash Flow Report
Key highlights
- Botanix is on track to launch its patient experience program in Q4 CY2024
- Botanix has finalised contractual arrangements with a number of key US payers (insurers) which reflect the target financial and patient access restrictions previously communicated, with
- further contracts to be signed in coming months
- Botanix has filled key positions in sales, marketing, sales training and sales operations that will
- support the commercialization of Sofdra
- In September Botanix was featured in both the ASX Small and Mid-Cap Conference and the E&P
- Small Cap Healthcare Conference in concert with a non-deal roadshow
- Cash position of A$67.62 million at September 2024 quarter end with no debt
Full Ann click here
At a glance there is nothing exciting here, but that's fine. Seems to be on track. In line with the plan.
Staff hired and rolling on toward sales in the new calendar year.
Steady as she goes.
Wow out old left field- I am just digesting how this news could impact Botanix’s value.
There will be a senate inquiry into Pfizer and Eli Lilly’s online telehealth platform. Is there room for abuse and over prescribing or a breach of possible kickback laws for drug companies.
I need to do more reading but I wanted to highlight this and kickstart some conversations around this topic.
Botanix uses a telehealth set up and access to a doctor who can prescribe a medication for AHH. It then uses independent rx provider Upscript to dispense this medication. Does this provide Botanix with an arms length protection? Does this protect the overprescribing of said drug and Medicare / Medicaid rebates that the government is worried about in the US. Or does this threaten all online telehealth platforms. A must watch piece of the puzzle for Botanix holders.
This could potentially add a lot of value or place uptake pace in danger if this distribution plan is vetoed by senate.
My gut feeling is that Big pharma running telehealth and distribution networks themselves may be vetoed.
Let me know your thoughts.
Probably no new information here for established BOT enthusiasts, but I found this short interview with Howie McKibbon a snappy summary of the current state and year ahead:
Hiring announcement out this morning Click here
At first glance they seem to be hiring some decent talent, who have runs on the board.
The hiring of John Walsh for example is to some extent getting the band back together:
- John Walsh – Vice President, Sales – Mr Walsh was most recently Regional Business Director for Dermavant Sciences (a company previously managed by Mr Ippolito and Botanix CEO Dr Howie McKibbon, which was sold to Organon for up to $1.2 billion recently). Mr Walsh has held senior sales roles with a variety of leading dermatology focused companies including with Anacor and Pfizer.
It seems to this layman that this is a team who have been here before.
Botanix CEO Dr Howie McKibbon's 20 minute presentation at the recent ASX Small and Mid-Cap Conference September 2024 is now available on Youtube
ASX Small and Mid-Cap Conference September 2024 | Botanix Pharmaceuticals Limited (ASX:BOT)
Here's Howie again at the small and mid cap conference. He does mention an AI driven marketing campaign - that's got to be a good thing right?
Botanix with Proactive at ASX Small and Mid Cap Conference
Botanix Ltd CEO Dr Howie McKibbon sits down with Proactive's Tylah Tully live from the ASX Small and Mid Cap Conference in September 2024.
BOT CEO Howie McKibbon - interview
https://ausbiz.com.au/media/botanix-sweating-on-sofdra-launch?videoId=37991
Botanix held a webinar to update the market on the commercial launch of Sofdra.
One of the key points I took away is that the number of diagnosed patients in the US is now thought to be 7 million out of 10 million people suffering axillary hyperhydrosis. Botanix previously had this at 3.5 million diagnosed. If correct that means there are twice as many patients who are aware of their diagnosis and open to trying a new product.
This may also increase the number of patients able to access Sofdra with no out of pocket expenses. If an insurer requires a patient to have already tried an alternative treatment before authorising Sondra, it seems having a larger cohort of diagnosed patients increases the number of patients who have already tried a competitor and be eligible for $0 out of pocket coverage. This could bring forward the customer adoption rate and accelerate sales.
Where to from here?
So far so good. I’m bullish on the prospects for Botanix, to the point where I’m asking myself: what am I missing?
There is execution risk: it’s possible that all these plans don’t work, or the product doesn’t work, or the customers try it and just switch back to competing products.
The thing is it’s not actually a new product and has been successfully sold in Japan (though a different formulation). So it probably does actually work. We’ll have to see how many patients try it and stick with it. Maybe it’s better than the alternative but not that much better?
If these commercial plans come off the upside is significant. I’d expect low multiples of the current share price and then up from there if customer retention is good.
So why does it appear to be overlooked? There’s no revenue yet, so the stock probably doesn’t look attractive on traditional metrics. You have to understand what is in motion. I also wonder if it suffers a bit from being in a less glamorous market: they aren’t curing cancer. This is OK with me but maybe makes it less exciting to follow for some.
The share price has had a good run up over the last 12 months, and I’m weighing up whether to buy more. I’m suffering from some price anchoring here - I bought it earlier when it was cheap, but if I do believe the price remains significantly undervalued then the current price is attractive with less risk than when I purchased previously. I probably will buy more but be patient. I don’t think there’s going to be anything in the next few months to cause a price inflection.
From Stockhead: Click here
Alive and Kicking: No sweat as Botanix readies for US launch of its hyperhidrosis treatment
Health & Biotech
Alive and Kicking is renowned biotech journo Tim Boreham’s new daily wrap covering morning movers and shakers of note in the ASX Healthcare sector, Monday through Thursday.
- Botanix plans a US launch of its anti-sweating treatment in the March 2025 quarter
- Pacific Smiles shareholders have a new offer to get their teeth into
- Bubs Australia enrols its 400th and final infant for US clinical trial
Botanix Pharmaceuticals (ASX:BOT) says it will launch its approved product for excessive sweating in the US in the March quarter of next year, targeting 10 million sufferers of the common but little-known primary axillary hyperhidrosis (PAH).
In June the US Food & Drug Administration green-lighted its topical gel Sofdra, a “novel, safe and effective solution for patients who have lacked treatment options for this socially embarrassing medical condition.”
PAH is sweating over and above what is required to regulate the body’s temperature. We’re talking about shirt-drenching sweat, not something that can be ameliorated with an extra spray of Brut 33.
It’s the third biggest dermatological condition behind acne and dermatitis.
At an investor update this morning, the company said it had recruited 500 patients via the International Hyperhidrosis Society – yes, there is such a thing – with 18,000 to follow.
Meanwhile, the company has hired 27 sales reps across three regions to kick-start the sales and is wooing the small number of physicians – 4500 or so – who prescribe PAH treatments.
The company says seven million PAH patients have been diagnosed over the past 10 years, while three million remain undiagnosed. Given the US population of 336 million, that’s an incidence rate of a not insignificant 3%.
The company stresses that Sofdra will be eligible for reimbursement, with no out of pocket costs for most patients.
Initially, Botanix focused on developing synthetic cannabinoid treatments for skin diseases including acne, atopic dermatitis and psoriasis.
But results were patchy and in May 2021 the company obtained the rights to the sofpironium bromide (renamed Sofdra).
Unlike antiperspirants, Sofdra addresses the underlying problem by suppressing underarm receptors. Technically, Sofdra works by inhibiting M3 muscarinic receptors in eccrine glands at the application site.
Found throughout the body, these receptors induce a ‘fight or flight’ response, including sweating and salivating, lactation and even urination.
The excessive sweating comes in three iterations: primary axillary (under the arms), cranio-facial (head and face), palmar (hands and palms) and plantar (feet). The company has FDA approval for the first.
Initially, the FDA rebuffed the company because of concerns about the wording of instructions in the Sofdra packaging, but these issues proved surmountable.
However the March 2025 timeline is a slippage from the originally envisaged mid-2024 launch.
Immediately after approval, Botanix raised $70 million in an institutional placement, so it is well placed to lob its first US underarm delivery.
Botanix shares closed one cent higher at 42 cents, ascribing a meaty $770 million market capitalisation.
$BOT have posted the presentation for their Commercial Webinar later this morning at 10:30am.
Good clarity on the timeline and how different US market segments will be accessed.
The Feb-25 HY report should give comprehensive (??) feedback from the Patient Experience trial, as well as the initial progress on IHhS (18,000) and Targeted Patient Lists (1 million).
The Feb report will be very significant, as it will provide decent real world commercial sales data from which forecasts will be updated. I expect there will be huge divergence that early in the s-curve, given the conservatism of current forecasts! That is, unless the product bombs!
Very much looking forward to the next 6 months.
Disc: Held in RL and SM
Some back ground info regarding suffers of Hyperhrdrosis from the International Hyperhrdrosis Society Website (sweathelp.org).
It’s quite eye opening to see the impact this can have on one’s life.
Key Facts About the Hidden Costs of Hyperhidrosis
- There are approximately 15.3 million individuals living with hyperhidrosis (Hh) in the U.S. (Archives of Dermatological Research 2016)
- Hyperhidrosis' impact on quality of life is equal to or greater than that of psoriasis, severe acne, Darier's disease, Hailey-Hailey disease, vitiligo, and chronic pruritus. (Eur Med J Neurol 2001)
- The prevalence of anxiety and depression is significantly higher in those with Hh than those without Hh (21.3% vs 7.5% and 27.2% vs 9.7%, respectively). (J Am Acad Dermatol 2016)
- 20% of hyperhidrosis sufferers report problems using computer keyboards, a computer mouse, mobile phones, and touch screens. (Health and Quality of Life Outcomes 2017)
- 80% of Hh sufferers say they are dissatisfied with their abilities at work; 42% say that Hh prevents them from following a particular career path; 30% say they become frustrated with daily activities. (Brit J Dermatol 2002 & Dermatology 2006)
- Hh sufferers have a 300% greater risk of skin infections. (J Am Acad Dermatol 2009)
- 60% of Hh sufferers report negative impacts on general health. (Archives of Dermatological Research 2016)
- 40% of Hh sufferers report physical discomfort. (Health and Quality of Life Outcomes 2017)
- 5% of Hh sufferers indicate they take antidepressants or anti-anxiety medications due to their sweating. (Brit J Dermatol 2002)
Came across this post on Livewire where Lennox Capital fund manager discusses Botanix as a high conviction position:
With binary approval risk in the US no longer being an issue, we think the opportunity in front of the company is immense, especially when you couple that with management’s history of commercialising dermatological products in the US.
I don’t know Lennox Capital at all so don't put any weight in their picks as such, but I do find it interesting to see a fund manager declaring they are bullish, and something to watch for more momentum in the price. Trading volume still higher since the FDA approval but is easing.
informative article in end points https://endpts.com/next-gen-biotech-acquirers-find-ma-momentum-in-q2/
Take overs/ buyouts for 2024- looks to me like there is some room for Dermatology and Neurodevelopmental drugs - nothing on the list so far !


As @Strawman made special efforts to feed the "spreadsheet jockeys" on the call this morning, I thought it only fair to respond in kind.
Matt referred to the potential that Sofdra really takes off, being a case not at all considered in the E&P and EH valuations of $0.47 and $0.55. So I thought I'd have a go a putting some dimensions around that.
Assumptions (details shown in the spreadsheet below)
- Assume sales build to peak over 5 years according to the profile given
- 5% of diagnosed market captured in yr 5
- 2.5% of undiagnosed market capture in yr 5
- 12 scripts per year at 50% persistency (absolute, not progressive/cumulative)
- %GM 76%; SG&A grows at 20%; R&D at 10% of revenue; nominal DD&A (capital light)
- No debt
- SOI grow at 2.5% per annum
- P/E in 2029 of 50 (which is modest if this baby takes off)
Crank the handled and discount back at WACC of 10% and you get a valuation of $12bn, today.
In this scenario, they are writing 2m scripts p.a. in 2029. That's just double the rate in Japan after 3 years. With excellent marketing and execution, that's not inconceivable.
To be clear, this IS NOT my valuation. I am happy to leave my valuation at a "modest" $1.20.
The point is that if we see a strong revenue trajectory in 2Q25, 3Q25 and 4Q25, then this indicates the order of maghnitude change that could possible emerge - which is probably more in line with where Howie and Vince are thinking.
For sure, there is a lot amount of execution risk. But this kind of upside potential means that this morning I've added a further 20% to my RL holding at $0.335, and will align SM accordingly. I want to place a bigger bet here.
I wasn't around to get on the $PNV bus pre-revenue, but I'm damned sure that I'm on this one with a solid position.
I'll write a separate straw with some reflections on the SM meeting later today.

Disc: Held in RL (7%) and SM
The recording for today's meeting is up, but here are some notes:
Botanix Pharmaceuticals, founded in 2016, spent a bunch of time and money looking for suitable applications of the Permetrex drug delivery technology, and relatively recently hit upon an treatment for primary axillary hyperhidrosis (excessive underarm sweating) -- a product called Sofdra that has just got FDA approval and for which there are 10 million estimated sufferers in the US alone.
For those affected, there aren't a lot of good options (existing options range from antiperspirants to invasive procedures like Botox and nerve surgery.)
But this product, which needs a script from a dermatologist, and for which should have minimal cost for (insured) patients, has the potential to be a bit of a game changer.
The company is now looking to gear up to meet expected demand (FDA approval of marketing materials, distribution setup, and launching a patient experience program etc) and expects first revenues later this calendar year.
Targeting early 2025 for sales force deployment. The company is hoping to bypass a lot of traditional pharmacy challenges with direct-to-patient distribution. And aiming for minimal out-of-pocket expenses for patients through insurance negotiations.
BOT is projecting market share of 30% for diagnosed patients and targeting undiagnosed patients through digital and telemedicine efforts.
Focus is on the US market due to high pharmaceutical spend. But exploring partnerships for other markets (e.g., Japan, Australia, Europe).
The team behind the company appears to have serious form, with people like Vince Ippolito (Executive Chairman) and Howie McKibbon (CEO) demonstrating successful track records in dermatology product launches and exits.
Execution on current plans while exploring additional product opportunities.
Potential for expanding Sofdra™'s use to other body parts.
When asked about the potential financials, Matt provided the following insights:
- In the US, there are 10 million people with underarm hyperhidrosis.
- 3.7 million have been diagnosed in the last 12 months.
- A typical new product aims for a 30% market share of diagnosed patients within a few years.
- Targeting 1% of the remaining 6.3 million undiagnosed or non-recently diagnosed patients through digital and telemedicine efforts.
- Each patient using the product 12 times per year.
- A target net revenue of $500 USD per patient per month
In his words, that's a "very big number" -- if my math's is right about US$7billion per year (of which some will be taken by contract manufacturers and other counterparties.)
Matt referenced the following notable exits during the interview and expressed confidence in Botanix's potential to achieve similar outcomes:
- Anacor Pharmaceuticals (Vince Ippolito, the Executive Chairman of Botanix, was the lead commercial officer at Anacor) developed several products, and was acquired by Pfizer for $5.2 billion.
- Medicis Pharmaceutical (Vince was also a senior commercial officer here) was sold for $2.4 billion.
Matt said the team would be disappointed if they didn't achieve a similar outcome.
Of course, there's a lot of execution risk still present, but it does seem like the company is positioned well.
$70m at $0.30 for 233.333m shares - a dilution of c. 15%.
No SPP as suspected.
Use of funds focused on Sofdra.
Thanks to the loyal shareholders.
While it’s easy to be disgruntled, the big picture is that not too far down the track, this business is likely to be worth a lot more.
Overall, I do want this business to have a strong balance sheet, but I still don’t understand why they didn’t raise a few days down the track.
Will look at the price action at the open and see if I can sneak in some more. Unlikely though.
While arguably smart, it's also a little sneaky. Botanix won't be coming out of their trading halt as there will be a capital raise. It will be very interesting to see the details around this. I'm not goint to lie though, I'm disappointed not to see the pop in the share price this morning.
$BOT has announced approval of Sofdra.
(Just seen the ASX announcement come through as I board flight for 2 weeks holiday.)
Just over a month ago Euroz Hartley published a short term price target for $BOT of $0.33, predicated on their assessment of the typical run-up in SP ahead of an FDA decision. Sure enough, with days to go, SP has passed this target standing today at $0.345.
I have absolutely no interest in short-term trading, as I am placing a 90% risked bet that this will be a much more valuable proposition. Much more over time. That said, it is interesting to watch the volumes and prices.
Last week's "non-announcement" appears to have given the market a little nudge, reminding the hot money out there about the impending FDA decision.
In the success case, on fundamental grounds, I expect the SP to go a lot higher. However, if there is a negative decision, this baby has pumped up enough that any correction will likely be hard indeed.
At time or writing there are still slightly higher volumes in the "BUY" queue (9m) than the "SELL" queue (7m), but things have evened up a little from this morning. But today marks the 3rd conseuctive day of higher than normal volumes.
tick-tick-tick
Disc: Held in RL and SM

Botanix has successfully raised $13.5m via institutional investors in preparation of approval and a launch of their 'Sofdra' product in the USA.
Unfortunately management have made no mention of a retail shareholding placement. I always find such scenarios ridiculous and speaks of the arrogance and contemptuous of management towards their retail investors when capital raising aren't also offered to them. While personally this won't affect me as I already have my fill and would not have participated myself, I feel others in my position should have been offered the opportunity to obtain more shares at the discounted rate. Currenlty the share price is still hovering around $0.15 while institutional investors accessed a price of $0.13.
$BOT are holding their AGM today.
The AGM Presentation has no material new information, but it is a good overview of where they are at. Next milestone is meeting before year end to get FDA guidance on the labelling/PIL feedback that has delayed approval of SOFDRA until mid-CY24.
Presentation has further details on the sales and marketing strategy, including details on sales force, geographic focus and telehealth strategy (recent partnership with UpScriptHealth annoucned separately).
Disc: Held in RL and SM
I wanted to better understand the precedence around the FDA letter holding up approval of sofpironium bromide gel based on labelling and patient information deficiencies.
I found an interesting reference on the subject in JAMA (Sacks et al. (2014) JAMA 311(4) 378-384). The article is a little too dated for my liking, but the information in Table 5 is interesting. (below)
Of 151 drugs not approved in their first cycle, 71 were subsequently approved following resubmission, with 80 not approved during the lifetime of the study.
What is more interesting is that only 4 drugs (2.6%) were not approved first time for labelling alone, and all 4 of these were subsequently approved.
Although the median delay in the study was 435 days this includes the entire population which is dominated by drugs with safey, efficiacy, chemistry, manufacturing and controls issues, many of which would have required further clinical trials. There's no data on the delay for labelling ony.
So this provides some independent support for the CEO's confidence that the drug will be approved when the labelling feedback is addressed.
It is many years since I was a practitioner in the industry, so it is good to know that the 2.6% of labelling rejections aligns with my own intuition that, while not un precedented, a labelling-only knockback is unusual.
In his interview on Ausbiz yesterday Howie McKibbon said "It's something that we anticipated. If we were going to get any feedback from the agency, we would have that much earlier in the cycle. So we were quite surprised that this occurred," which reinforced my own expectation that label feedback is usually addressed in earlier communication between the FDA and applicant, prior to the final decision.
Thinking about this overnight, I think the reason that this didn't happen in this case, is that the instructions relate to the use of the gel applicator, that requires validation in a controlled environment. So, its not just a change to the label (as you might easily do for a pill or capsule) but a patient instruction that has to be validated by observation and requires a supplemental submission reporting the results. I think that is why a resubmission is required and makes me think that maybe Howie shouldn't be so surprised. (It also weakens any argument about conspiracies!)

Source: Sacks et al. (2014)
The big question mark I have around this one is why did Freshtracks agree to close out the licence aggreement for what can only be described as a steal for Botanix. At the time that Botanix bought Sofpironium Bromide they described it as a "Deal structured with minimal upfront payments, with most of the consideration payable in Net Sales milestones and royalties". The recent agreement to close out the licence royalties and milestone payments really doesn't make any sense if you were FreshTracks. FreshTracks were due to get $4m payment when/if the FDA approval is received in late September, so closing out all of the future payments (>$160M potential) for $8.25M seems ludicrously shortsighted by them. This makes me question why would they do this as presumerably there is a good reason. FreshTracks aren't flush with cash but they are also not desperate either and as of March 23 they had $10M in the bank which they said will cover them for the next 12 months. I am also wondering why no other large pharmaceutical company wanted to either buy the rights to Sofpironium Bromide or to buy the potential royalty stream from FreshTracks. I am a bit sceptical about this deal as it seems incredibly onesided in Botanix's favor. Given that until Botanix stumbled upon this drug in early 2022, and prior to that they were trying to develop skin creams from cannabis for achne and rosaca, which failed in phase 2 trials.
This all makes me suspicious that FreshTracks could be the better poker players, and they know/suspect something (as the drug developer) that could become problematic during its market rollout. They have accrued the development costs and are now effectively giving it all away for a total of $13.25m. I doubt this would have recouped their cost of the development of Sofpironium Bromide.
The final nail for me was reading the interview with Alan Kohler where the CEO described the phase 3 results of Sofpironium Bromide as "pretty highly statistically significant". Significance either is or isn't at a specific level, but can't be highly or slightly.
If the drug is legit and Botanix are able to charge $700/ month for the initial target of 300000 paitents with a 5-10% COGS then the financials look impressive and they should do very well, but I have an uncomfortable feeling that this isn't the most likely outcome or the full story. Good luck to holders but I think I will watch this one from the sidelines.
I am reminded by that saying that if you can't see the sucker at the table then it is probably you.
If SB is approved in September and with a forward looking market $100 million sales revenue is potentially achievable. This is based on Kaken’s current sales 300,000 units. So very conservatively if half those units are sold at say $700 a year per patient. 150000 x 700= $105 million rev.
Assume a rounding error make it $100 million revenue year 1 of sales (remember US has 3 x population of Japan. Take a PE of 50 (conservative) for a ASX biotech stock.
$100 million revenue less the cost of manufacture, marketing, shipping etc…this is the big unknown so say earnings are 25% = $20 million/ 1.3 billion SOI (shares on issue) x PE 50 = 80 c
Say earnings in the first year has a 50% margin so $50 million /1.3 billion x 50 = $1.92
Food for thought.
All other products rosacea , acne and anti-biotic if pass phase 3 and get approval would have at least this earnings potential likely much more as HH is only the 4th biggest dermatological market. So exclude antibiotic as biggest long shot and you still have 3x $1.92 = $5.76 SP so I think an offer from big pharma needs to be pretty enticing.
For completeness:
The ultimate research question is why Brickell Biotech sold Sofporonium Bromide to Botanix if the drugs is a) so great b) so potentially profitable? Why wouldn’t they take this drug through FDA approval themselves.
I think both Botanix and Brickell needed something from each other at the right time.
Botanix needed a commercial product to add to its pipeline – I think COVID added a legitimate time delay to Botanix’s original pipeline plans.
A faster pathway to revenue generation would help the market to regain faith in Botanix and hopefully increase the share price and investor returns. A commercial drug plus a phase III and multiple phase II drugs is a much more attractive buyout target for big pharma than just a clinical company.
Brickell Biotech was a small US clinical stage company. Brickell listed on the NASDAQ in 2019. Prior to this it was valued between $50 to $100 million privately in 2015.
Brickell underwent 5 funding rounds totalling $33.5 million. The biggest investor were Amorepacific Ventures, Charlie Steifel and Pallisades.
2010 Funding Round 1 - $6.2 million
2013 Funding Round 2 - $7.0 million
2014 Funding Round 3 - $2.2 million
2015 Funding Round 4 - $10 million
2021 Funding Round 5 - $8.1 million
Bodor Labs licenced SB to Brickell and in 2015 Brickell licenced SB to Kaken for Japan and parts of Asia. Brickell got upfront payments and milestones and tiered royalties which were undisclosed.
2015 also saw Brickell expand its drug offerings into Posoriasis and Auto-immune disease. In 2019 Brickell acquired Vical for its DNA delivery tech for an undisclosed amount. Needless to say Brickell was a clinical stage company relying on funding rounds and small licensing revenue for SB from Kaken.
Nasdaq listing was successful and saw Brickell Biotech’s (BBI) SP soar. Positive Phase IIB results for 15% SB impressed the market and the stock hit $3.39. COVID affected biotech badly. In October 2020 the stock price was down to 0.56c and in Nov 2020 the Brickell CFO resigned.
Final funding round in 2021 for $8.1 million kept Brickell afloat. In Sept 2021 Brickell bought another drug DYRKA program in clinical stages.
The stock price dropped again and by May 2022 it was as low as $0.29c. The problem was that SB would take too long to get FDA approval so there was no immediate revenue possibility. I speculate that with Brickell collecting immunology assets and inflammatory assets that it needed to offload the most advanced sellable asset to inject some enthusiasm back into the share price and to fund the rest of the pipeline.
However the sale of SB to Botanix for $9 million upfront and $168 million in future sales and milestones did little for the Brickell share price. FDA approval would be 12 months plus away and so there would be a lag before any future revenue or milestone payments were achieved.
On June 30th 2022 it looks like the Brickell Board made a decision to do a reverse stock split 1-45 in an attempt to lift the minimum share price bid back above $1.00, as per NASDAQ compliance requirements.
After the stock split BBI returned to NASDAQ compliance in July of 2022 but that was short lived and Brickell again faced delisting in August, 2022. It was in September this same year that Brickell rebranded and announced a strategic shift into immunology and inflammation fields and away from dermatology.
I feel like as a clinical stage company Brickell was struggling financially during COVID like most of the market and BOTANIX came along at the right time with a chunk of cash and a possible injection of life back into the stock price which was ailing around and below the $1.00 minimum bid price.
I suspect Botanix ultimately ended up with the better end of the deal.
Botanix is now 2-3 months away from potential FDA approval and transition to a commercial pharmaceutical company. All just speculation on my part but I think we will look back at this time as a very strange era in biotech history. I think Vinnie was there with a nice price at the right time and Brickell looks like they needed this.
Competition to SB
Specifically I have dug into THVD-102.
I think with any investment research it is important to understand a drug’s potential future revenue and any drugs that might eat potential future market share.
To understand THVD-102 lets follow its journey. There are some very illuminating outcomes and familiar characters.
THVD-102 was initially developed by TheraVida Inc in 2016. TheraVida developed a proprietary patent protected product for the treatment of hyperhidrosis. The company ran a small phase 2 trial combining Oxybutynin (muscarinic receptor antagonist) and Pilocarpine (muscarinic receptor agonist). Importantly this combination of drugs was a possible step forward in hyperhidrosis treatments.
Phase 2 trials found that Oxybutynin + placebo treatment performed similarly to the combination therapy of Oxybutynin and Pilocarpine, however there was a statistically significant improvement in associated dry mouth symptoms for patients on the drug combined with Pilocarpine.
The severity of dry mouth side-effects stopped people wanting to take the Oxybutynin alone.
This was a desirable outcome, the combined THVD-102 drug was thought to be a potential oral treatment for hyperhidrosis and a competitor to Allergan’s Botox injections.
Enter Roivant Sciences Ltd a self-described next generation “big pharma” company proclaiming that its competitive advantage is that is adaptable and nimble. With a NASDAQ (ROIV) listing and current shareprice $9.70, Market Cap $7.4 Billion and 758.43 million shares on issue.
Roivant’s Mission statement:
“To improve the delivery of healthcare of patients by treating every inefficiency as an opportunity.”
Roivant’s Goal:
“To identify novel or clinically validated targets and biological pathways in areas of high unmet medical need. Then seek to acquire, in-license or discover promising drug candidates against those pathways or targets.”
Roivant Sciences Ltd has developed an agile “VANT” model. Vants are subsidiaries of Roivant’s Sciences Ltd. These Vants specialise in therapeutic areas including immunology, dermatology and oncology.
Dermavant founded in 2014 is Roivant Sciences -dermatology subsidiary. Dermavant’s mission is to advance clinical phase dermatology candidates and is willing to carry out preclinical work.
Dermavant’s flagship drug is VTAMA (Tapinarof 1.0% cream) which gained FDA approval for treatment of plaque psoriasis in adults, in May 2022. Dermavant purchased all global rights to Tapinarof (except in China) from GSK in 2018. They also assumed the responsibility for all developmental milestones owed to third parties. Roivant paid GSK US$330 million and agreed to run all phase III clinical trials for both psoriasis and atopic dermatitis.
Dermavant paid GSK $198 million for the preclinical drug with a further $132 million in developmental milestone payments.
VTAMA is also a potential treatment for atopic dermatitis in children and adults. The drug yielded positive phase III outcomes as a safe and effective treatment for adult and paediatric (non-steroidal option) for the treatment of dermatitis, in March 2023.
Dermavant has a history of buying pre-clinical drugs and running phase III themselves (presumably to get the drug for cheap). This has proved to be a winning formula for VTAMA through to Feb 2023 the company had sold 100,000 prescriptions, written by 8,600 unique prescribers for plaque psoriasis. Net product revenue for the December 2022 qrt was US$9.2 million.
Back to THVD-102 – In 2018 Dermavant bought the rights to TheraVida ‘s hyperhidrosis drug THVD-102 and renamed the drug RVT-504. This deal consisted of an upfront fee and milestone commitments of a undisclosed size. The plan was to run this oral drug through phase III trials and hopefully launch it as a competitor to Allergan’s BOTOX.
Fast forward to June 2022 and Pfizer partnered with Roivant Sciences in a deal to launch TYK2 inhibitor Brepocitnib for the treatment of dermatomyositis and lupus.
This was an expensive deal and meant that mothballing several Vant subsidiaries would be necessary. Dermavant lost half its dermatology program funding. RVT-504 for hyperhidrosis was cut. So this drug has not been developed any further.
Now to the interesting part. Howie McKibbon Botanix Pharmaceuticals current Chief Operations Officer most recently served as Senior Vice President for world-wide commercial operations at Dermavant from 2017-2019. Therefore he would have been very familiar with hyperhidrosis market as he was present at the time of the Dermavant purchase of THVD-102. He had not doubt realised this competitor had mothballed their program and would be years away from being a viable competitor to the more advanced SB program.
He would certainly be privy to Dermavant’s dermatological program and the fact that this company had a gap in both the acne and hyperhidrosis space.
Roivant Sciences has certainly been known to partner/ licence and buy rights to pharmaceuticals including pre-clinical drugs. I found it interesting that during recent meetings with share-holders there was a huge stress placed on Botanix being very happy to go to market themselves. Perhaps this was a signal that they would not be willing to take a low offer for a preclinical drug from the likes of companies like Dermavant. They are likely holding out for the big offers once the SB has FDA approval and sales. All speculation of course. DYOR.
Pls note the one and only time I have shared this on another forum first. I wrote this in response to a poster's question elsewhere. I have included this on Strawman for completeness of any and all analysis on Botanix Pharmaceuticals.
Botanix pharmaceuticals is no longer a pre-revenue biotech.
Botanix is entitled to royalties for sales of SB from Kaken Pharmaceuticals in Japan. Details are still vague on sales numbers and royalty amounts but will become clearer with the next few 4Cs.
Further news announced yesterday is that Kaken has struck a deal to sell SB into South Korea through Dong Wha Pharmaceuticals. Botanix will be entitled to royalties on sales in South Korea also.
While the company states the sales royalties will not be significant a new territory of SB distribution further derisks this drug. It increases likelihood of FDA approval in the US come September. This will be a direct sales and revenue path of Botanix.
Interesting article highlighting some fan favourites PME included but also mentions Neuren Pharmaceuticals and Botanix Pharmaceuticals potential to be market giants if dominoes fall the right way. The elusive next CSL many of us are hunting for.
https://www.livewiremarkets.com/wires/is-another-csl-hiding-on-the-asx
#Botanix #FDA filing accelerated #2clinicaltrialsontrackforcompletionQ3
22/23 are shaping up to be pivotal years for this small Australian biotech. Currently sitting with a $68 million market cap and $17 miilion cash on hand.
I am a strong believer in this company due to the proximity to revenue and the range of potential products. This I believe diversifies the risk of my investment. The conditions targeted are common problems that have lacked innovation for decades. Often competitors such as acne drugs can have severe side effects (e.g. roacutane and suicidal ideation and intolerance to sun exposure). So far synthetic CBD is proving to be incredibly safe in all clinical trials.
There is a current Product Pipeline of 5 drugs for a range of conditions. 1 pending FDA approval and commercialisation.
Table 1: Current Product Pipeline

(Source: https://botanixpharma.com/pipeline/)
This week’s announcement was very positive for Botanix Pharmaceuticals next step toward revenue generation. It’s new drug to treat PrimaryAxillary Hyperhydrosis (excess sweating) Sofpironium Bromide (SB) filling for NDA status with the FDA has been bought forward to this quarter. There is a very high chance of FDA approval given that 85% of patients who used SB experienced clinically meaningful improvement in their condition during the Phase 3 trial. Plus SB is already approved and currently being sold in Japan.
If successful SB will be Botanix' first product to market.
Botanix announcement confirms commercialisation preparations are already underway. Vince Ippolito President and Executive Chairman released this statement:
“over the coming months Botanix will commence the process of preparing for inspection of its contract manufacturing site and other FDA pre-approval activities.”
Furthermore Rosacea (BTX 1702) Phase 2 Clinical Trial is fully recruited and underway. This clinical trial is crucial in advising synthetic CBD dosage that will be used in upcoming Phase 3 Acne trial.
The canine dermatitis pilot study (BTX 1204A) is also fully enrolled and due for completion in Q3. This will advise whether future human dermatitis trials will be pursued and provide another quick to market commercialisation opportunity if successful in the veterinary market.
No doubt many of us are finding it harder to find decent returns in the current markets. I know as someone heavily invested in health care and the tech space my IRL portfolio has seen prettier days.
With the health care index tipping as low as -12.68% compared to the benchmark S&P ASX 200 you can be forgiven for looking away and going to the pub.
However there are some bright spots in amongst the carnage. Kalkine media has just released an article highlighting the best year to date returning healthcare stocks https://kalkinemedia.com/au/amp/news/stock-market/from-imc-to-ala-healthcare-stocks-with-best-ytd-returns
It will be no surprise to current Strawman holders some of the better performing stocks these include:
IMC
Neu
LCT
BOT
ALA
I currently hold Neu and BOT IRL and on Strawman.
Neu has had multiple major milestones over the past 6/12. Its major lift was toward the end of last year however positive momentum has continued in 2022 with YTD returns of 7.96%. Share holders are patiently waiting for the second company inflection point in June this year. Shareholders are optimistic of FDA approval of its drug Trofinetide for the treatment of Rhett Syndrome after a successful Phase 3 study. I am keen to stay invested for this as well as a possible rest of the world deal for this same drug- this could be announced anytime.
BOT has seen even better YTD returns of 41%. Management has been actively recruiting and made recent high caliber hires to join its Board to help with upcoming commercialization. A new Non-Executive Director position has just been filled by Danny Sharp - who has 30 years experience in commercial banking with specialties in healthcare and technology. He also currently sits on the Board of Alcidion (ALC).
The CEO and President of BOT have been hitting the pavement at recent roadshows eluding to a likely upcoming commercial deal with an expanded product line involving their patented skin delivery system Permatrex. They are actively seeking assets to acquire that can quickly and easily utilize the delivery system and be ready for immediate commercialisation. This may provide a revenue stream much quicker than shareholders had been expecting.
It is nice to hold stocks with plenty of cash in the bank and further upcoming inflection points.
Biopharm magazines have frequently mentioned the number of cashed up pharma companies that are looking to buy startups with progressed assets. In an inflationary world that is becoming increasingly difficult to get access to capital, I see the biopharm sector as being a promising anomaly.
Botanix Pharmaceuticals Management
24.09.21
Botanix has announced the expansion of their Senior management team with the appointment of a new Senior Vice President of Pharmaceutical Development Dr. Jack Hoblitzell PhD.
The nay sayers could potentially argue this is a sign of bloated management expenses for a company that currently has no revenue outside Australian Government R&D grants. However, it all depends on the calibre of the new recruit. Botanix has been reasonable with cash flow management and managers keep salaries low and opt for future options in their packages.
Botanix has made no secret of future strategies to add quick revenue streams. The following angles are being pursued:
1) BTX1204A for canines and
2) Leveraging its exclusive lease of the ‘Permetrex’ delivery system (alcohol free system to improve penetration of drug molecules through the skin).
A quick LinkedIn stalk and review of Dr. Hoblitzell (why is some random chic from Australia looking at my profile?) suggests that he is a very appropriate hire at this point for the company.
Dr. Hoblitzell BIO
The press release promoted him claiming he has experience leading world class technical operations to deliver new product launches (tick).
Years of experience with regulatory submissions – wrangling FDA (tick).
Excellence in manufacturing and supply chain management. If anyone else was around for the great acne debacle of 2019 you would get this! CBD was a restricted drug in some states in the US and could not be transported across state lines…..nightmare. (so tick tick).
Experience supporting pipeline development and ACCELERATING the development and SCALE UP for commercial capabilities (tick tick tick).
Dr. Holblitzell is a registered pharmacist.
Where has he come?
I note a lot of Pfizer ties revealing themselves for Botanix Pharmaceuticals.
Formerly:
Snr Vice President Of Technical Operations at Assertio Therapeutics Inc. May 2020-June 2021 (not there long). Assertio Therapeutics has a $0.04 Billion dollar market cap. This company specialized in pain relief drugs: diclofenac potassium, indomethacin, oxycodone Hcl.
Dr. Hoblitzell has a fairly long history of overseeing mergers of pharmaceutical companies. This is also a point that Vince Ippolito CEO has frequently addressed that Botanix is a likely buy out target with the number of product pipelines they are developing. Frequently, referencing Jazz pharmaceuticals buyout of GW pharmaceuticals for $7.2Billion for their CBD based epileptic medication.
Of course everyone invested or researching Botanix Pharmaceuticals is well aware of our illustrious CEOs background. One of the main reasons I have stayed invested. He oversaw the Pfizer buyout of Anacor Pharmaceuticals dermatology platform for $4.5Billion.
Dr. Holblitzell has also been Senior Director of Pharmaceutical Technology and Global Manufacturing Services at Pfizer. Here he is endorsed by several Pfizer colleagues. At Pfizer he was responsible for the integration of King Pharmaceutical products and processes into the Pfizer co-development and manufacturing supply networks.
He was also Vice President of Technical Operations at Zyla Life Sciences for 5 years.
He was endorsed by former colleagues from King Pharmaceuticals where he worked before a buyout by Pfizer.
Lynn F Palmer Senior Vice President: Technical Operations of Pharmaceuticals at Osmotica Pharmaceuticals (Worked together with Dr. Holblitzell at King Pharmaceuticals) wrote:
“Jack is a practical and effective Pharmaceutical Development Executive. He has effectively led the development of multiple formulations, and scaled them up to commercial scale. He has extensive industry and technical knowledge of formulation development, product stability, pharmaceutical packaging and equipment capability and setup. I strongly endorse him. “
Pfizer’s brief
A pharmaceutical company that applies science and global resources to improve health and well-being at every stage of life. Pfizer has a $246 Billion market cap.
Interesting to note Pfizer looks to “set the standard for quality, safety and value in the discovery, development and manufacturing of medicines for people and animals.” (synergy with animals here as well)
Summary
He seems like a great get.
A lot of tie ins with Pfizer for both he and Vince – is this the hope? A Pfizer buyout?
I am really curious as to why a po-dunk back water Perth company with a $64 Million dollar market cap, currently trading at $0.074 per share is attracting these recruits. I certainly have my thesis – the CBD molecule patents and FDA fast track approval status achieved for their anti-microbial platforms are certainly compelling.
On Tuesday 2nd of Feb 2021 BOT went into a much anticipated trading holt for the release of results of its BTX1801 Clinical Phase 2a study. Preceding weeks had seen BOT share price peak at 18c around the 25th of January in anticipation of results. This was a far cry from the 29c the company reached on 2nd of August 2019 on anticipation of a less mature stage of the company without the anti-microbial drug potential in the mix.
The share price leading into the anti-microbial BTX1801 announcement was also a much bigger discount to the Cowan investment of 20c per share made by the US company in 2019 in the lead up to BTX1503 acne results.
As investors are aware following mixed results of BTX1503 acne in 2019 the share price more than halved to 12c on October 25th. It continued its gradual decline to as low as 2c around March 2020 before a slow climb back to its recent 18c peak prior to the anti-microbial results.
Results released on the 3rd of February 2021 were excellent. Primary end points of the phase 2a study were met. The CBD BTX1801 gel and ointment formulations both successfully and safely reduced Staph infections in the nasal cavity of patients verse a vehicle control. A collective shareholder sigh of relief and anticipation was warranted.
Mr Market had a very odd response to this result.
Each clinical trial stage passage potentially de-risks the drug and improves chances of success and future commercialisation. This process is analogous to climbing each progressive rung of a ladder. Share-holders would then rightly anticipate share price rises accordingly as each rung is passed in Biotech companies. However despite this result trading saw a closing price of 17c on the 3rd following the announcement and a gradual decline to 13c at close on the 8th of Feb, 2021. This price was near parody to the SP after the disappointing mixed results of the acne BTX1503 drug in 2019.
The share price does not currently appear to reflect a successful 2a BTX 1801 trial outcome.
Seemingly the market failed to acknowledge the passage up another rung of the ladder toward a potentially efficacious and safe new anti-biotic class with synthetic CBD BTX1801. This will be the first new class of antibiotics in 60 years. It was also the first human trial in the world to show that CBD can kill Staph aureus in 76% of patients after 5 days of treatment and that it has residual effects for up to 28 days.
This was a phase 2a study so optimal treatment dosages and comparisons to other drugs on market was not the study design. Safety and early efficacy and formulation methodology are being established. Nothing more nothing less. Was it safe, tick, was it effective, tick.
The meeting of the primary end points of this BTX1801 study can likely be extrapolated to provide further supporting evidence for the other drugs in the pipeline using synthetic CBD. Acne successfully kills staph aureus. Staph is part of the natural flora of the skin and overgrowth of this bacterial gets into pores causing acne. Ergo reduce bacterial load reduce acne.
Hence the results are another rung or two up the ladder toward successful commercialisation of potentially 2 pipeline drugs.
Biotech investing is a complex game and one where opportunities can be found and advantages can be had for those with a scientific background. Scientific language seems to be the area of confusion where market price can be won or lost or confusion and games can ensue to the advantage of those who know how to play. What do the primary end points mean? What are p values? What sample sizes carry statistical significance. Market misreads are certainly not unique and hesitation of market responses can be used to traders and investors advantages. However it can also lead share-holders to sell when they should hold or buy more.
Add to the complexity that traditional value investing rules do not apply when assessing a Biotech. Firstly there is rarely a commercial product producing income in early start up companies. R and D tax refunds are often the only revenue these early stage businesses rely on. Otherwise outside investment from bigger pharmaceutical companies or entities with a vested interest may produce some capital.
So why would an investor take on the risk of investing in the Biotech? How does one even start to value or understand the market capitalisation of these kinds of companies when traditional metrics are out the window?
Each investor has their own reasons and motivations: some believe in the science or the potential life altering outcomes of the new therapeutic or diagnostic test being developed while others know that the biotech space is traditionally a high risk and high reward play.
Biotech companies rely on their shareholders to provide working capital to progress from pre-clinical through to often costly phase 3 trials.
I believe value comes from novelty, the problem being solved, the need and the potential addressable market and the number of products in the pipeline of the Biotech.
So rather than looking at balance sheet and revenue growth the incremental value increases occur with the rungs climbed up the ladder from the pre-clinical proof of concept through to the Phase 1 safety through to the Phase 3 results. All are significant milestones for these companies.
90% of drugs fail to reach commercial products due to safety issues. Botanix Pharmaceuticals has a SAFE synthetic CBD product across all its drug channels: acne, anti-microbial and rosacea.
In a study of 640 phase 3 novel therapeutics 54% failed in clinical development. So even if a drug makes it to phase 3 less than half will be approved to commercialisation. 57% of those drugs will fail due to inadequate efficacy (Hwang T.J., Carpenter D., Lauffenburger J.C., Wang B., Franklin J.M., Kesselheim A.S. Failure of investigational drugs in late-stage clinical development and publication of trial results. JAMA Intern. Med. 2016;176:1826–1833). BTX1801 reduced Staph infections and destroys the bacteria through destruction of the cell wall. This drug potentially can be used without Staph aureus building resistance to it (lab tests only so far).
BTX 1801 anti-microbial once again has shown to be safe, tick, effective in 2a trials, tick. Given its tolerability and safety profile there are low drop out rates in previous trials and likely to be compliance of participants in trials moving forward. The management team also has experience with following FDA study guidelines given their vast experience at previous companies. Failing to comply with FDA study regulations are another reason trials frequently fail.
I perceive the biggest likely hurdle for this company is not failure in trials due to efficacy, safety or not following FDA regulations. The biggest risk given COVID is recruitment and trial interruptions moving forward. Hopefully this can be mitigated or future trials can be continued in Australia and New Zealand as opposed to the US to ensure continued timely progression of the companies pipeline.
Botanix pharmaceuticals is now a company with diverse and maturing product pipeline with a 2b Rosacea trial about to begin Q1 2021. This addresses a unique market in its own right and simultaneously provides an FDA building block toward Phase 3 BTX1503 acne the final phase prior to commercialisation. Further anti-microbial BTX1801 advancement should be fast tracked and phase 2b trials should also begin this year.
Given this companies progression up the ladder I do believe it is currently significantly undervalued. Share-holders will stay and more will be added to the register to help fund future studies but reward is needed to incentivize new money into Biotech companies. That is the nature of the high risk high reward play. Opportunity costs can indeed be great if money remains parked in these companies without bouts of significant reward.
It is obviously up to the each individual shareholder to assess whether the progression of the drug pipeline of this company has merit.
In the next 2-3 weeks Botanix Pharmaceuticals is about to reach a significant clinical milestone in its drug development program.
Results of the Clinical 2a Antimicrobial BTX 1801 program is just about due and this will be a huge potential inflection point for this company.
Share price took a beating on mixed results for its acne phase 2b trial in 2020 seeing the Share price drop from a peak for 29c to lows of 2c.
Investor faith in this company has waivered. However the Australian arm of these results were incredibly promising. The poor result from the US arm was due to complications in formulation consistency in this country, who was not experienced in the development of the carrier solution Permatrix or in the manufacturing of synthetic CBD used. This issue has since been rectified by sourcing a consistent manufacturing solution for future trials in the US.
Botanix is developing simultaneous programs:
1) Entering phase 3 Clinical trials for acne BTX 1503 in 2021
a. Addressable market currently US$2.9 billion annually in the US
b. Botanix addressable market estimated to be US $4.2 billion annually and US $7billion by 2024
2) Completing Anti-microbial 2a testing and data readout Jan 2021
3) Commencing Rosacea BTX 1702 1b trial in 2021
a. Addressable market US$2.6 billion by 2025
Multiple drug development programs I believe somewhat de-risks investment in this company.
Optimism in the antimicrobial BTX1801 programs success is based on the efficacy of results in previous clinical phase 1 trials and new data recently supplied to the market. The mechanism of action of synthetic CBD has been identified. The drug effects the cell wall of gram positive bacteria allowing penetration and eventual cell death of the bacteria without any apparent development of resistance to the new anti-biotic. This has been shown to eradicate MRSA (Methicillin resistant Staph Aureus bacteria) in both Porcine and Human Invitro studies (human stomach skin harvested from recent abdominoplasties). MRSA has been found to be eradicated completely in a 24 hour period after application of the new formulation.
Large groups of the population are known to carry MRSA nasally and anally. Self- infection is the route cause of most Surgical Site infections post-surgery. One of BTX1801s major target markets will be post-surgical site infections (SSI). The current anti-biotic most commonly used in SSI control is Bacitracin / mupirocin but it is increasingly ineffective and useless against MRSA.
WHO (World Health Organisation) has forecast deaths due to anti-microbial resistance to hit 10 million p.a. by 2050. This will overtake expected deaths by Cancer at this point. Estimates suggest that SSI cost the health care systems globally around US$10 billion dollars annually. The addressable market for SSI is estimated to reach $US 5.9 billion annually by 2023. This is a huge market for any new successful anti-biotic to target.
If successful BTX1801 will be a leading new drug in SSI. No new class of anti-biotics have been successfully developed in the past 30 years. This drug will have first mover advantage plus FDA fast tract QIDP status. Which means fast track of development programs to market plus 5 year exclusivity and a further 5 year protection of markets to competitors.
Human studies have now been completed, where BTX 1801 was applied nasally over 5 day treatment period in healthy subjects vs a control. Patients were followed for 28 days to see if there was reduction or complete eradication of MRSA. Successful outcomes should see a significant re-rate and restoration of faith in Botanix Pharmaceuticals simultaneous programs.
Investment in the Acne program alone saw US international investment at 20c per share in 2020. The additional anti-microbial programs success should see this price plus a significant increase based on the huge potential addressable market of SSI. Furthermore Anti-microbials will have other addressable markets – animal anti-biotics etc…..
The incredible safety profile of this drug will also solidify its game changing status on a global stage.
The current CEO Vince Ippolito has been responsible for negotiating significant buy outs of dermatological companies by Big Pharma. He was at the helm of Anacor pharmaceuticals when it was bought by Pfizer for $US 5.2 billion. There is a likely buyout potential for Botanix Pharmaceuticals on successful BTX1801 trials. Or at a future stage with further progression of its simultaneous Rosacea and Acne drugs.

